Infectious Diseases Institute, Gilead Sciences, hydroxychloroquine, TOGETHER Trial, genetic medicine
Originally published on Substack for Rounding the Earth
Early life and education
David R. Boulware was born in 1974. He attended Wabash College where he pledged Phi Kappa Psi. His fellow fraternity brothers would later describe him as “intelligent, humble, and well balanced,” and “able to balance academics, fraternity life, and athletics, too, as a member of the cross country team.”1 He graduated in 1996 with a Bachelor of Arts in Chemistry.2
He later attended the Indiana University School of Medicine, graduating in 2000.3 He completed an internal medicine-pediatrics residency and an infectious disease fellowship from 2000-2007 at the University of Minnesota, receiving a certificate in tropical medicine from the Centers for Disease Control and Prevention (CDC) in 2006. He completed his Masters in Public Health in 2007 in Minnesota.
Career
In 2007, Boulware became an Assistant Professor of Medicine in the Division of Infectious Disease and International Medicine at the University of Minnesota’s Department of Medicine.
As one of his first projects in his new position, Boulware began working within the university’s Center for Infectious Diseases and Microbiology Translational Research (CIDMT) under the leadership of Dr. Paul Bohjanen. Founded in 2005, CIDMT’s stated mission was “to discover mechanisms of pathogenesis that can be translated into new treatments and strategies for prevention of infectious diseases.”4
More specifically, Bohjanen and his team were one half of a long-term collaborative effort between the University of Minnesota and medical researchers in the African nation of Uganda. A 2008 article in MinnPost provides a summary of the origins of the collaboration:5
“The story began in 2003 when Dr. Paul Bohjanen came to Kampala to help train African physicians in the use of HIV medicine. Bohjanen brought the wisdom of experience because he treats HIV/AIDS at the University of Minnesota Medical Center. He also co-directs the university’s Center for Infectious Disease and Microbiology Translational Research. …
In Uganda, Bohjanen met the full brutal force of the HIV pandemic. It had killed nearly a million people, one in every 27 Ugandans. A million more were living under its death sentence. Their only hope for a reprieve was in HIV drugs, which were just beginning to trickle into sub-Saharan Africa. …
Back in Minneapolis, Bohjanen recruited a colleague, Dr. David Boulware. The University of Minnesota’s Academic Health Center contributed $200,000. More funding came from a philanthropic arm of the company Tibotec and the U.S. National Institutes of Health.
Boulware and Bohjanen boarded planes for Kampala two or three times a year, staying two to four weeks at a time. Students and fellows from the university’s schools of medicine and public health filled gaps between their shifts.

Of note is the fact that the MinnPost article acknowledges that “reporting for this article was supported by the Pulitzer Center On Crisis Reporting in Washington, D.C.” The Pulitzer Center is itself funded by a number of organizations who may find it in their interest to influence the way in which infectious diseases in Africa is covered in the media, including:6
- Bill & Melinda Gates Foundation
- Meta Journalism Project
- Nuclear Threat Initiative
- Omidyar Network
- Open Society Foundations
- Planned Parenthood
- Population Services International (PSI)
- Rockefeller Brothers Fund
- Rockefeller Foundation
- United Nations Foundation
- William and Flora Hewlett Foundation
…among others. It may be, of course, that the Pulitzer Center had a different, less conflicted set of donors back in 2008.
As described in the above article, Boulware and Bohjanen would travel to Uganda “two or three times a year, staying two to four weeks at a time,” where they would conduct research on the genetics of HIV/AIDS. They would also run clinical trials on various therapeutics on sick patients at the partner hospitals of the Infectious Diseases Institute.
Infectious Diseases Institute

The Infectious Diseases Institute (IDI) operates out of Makerere University in Kampala, Uganda.
It was “conceived” in 2001 by a group that included noted HIV/AIDS researcher Merle Sande, Makerere University School of Medicine Dean Nelson Sewankambo, and Hank McKinnell, former CEO and Chairman of Pfizer.7 This led to the formation of a non-profit organization called the Academic Alliance for AIDS Care and Prevention in Africa, or simply the “Academic Alliance,” which then set about founding the Infectious Diseases Institute in 2002.8
Initial funding for the project came from Pfizer, distributed through a separate foundation called the Pangaea Global AIDS Foundation.9 Within the span of just a few years, the IDI would develop strong relationships with American institutions including Johns Hopkins University, the Infectious Diseases Society of America (IDSA) and, of course, the University of Minnesota.10
As previously noted, Boulware and Bohjanen’s work with the IDI was funded by the University of Minnesota, the National Institutes of Health and a pharmaceutical company called Tibotec (a subsidiary of Johnson & Johnson which would later become Janssen Therapeutics).11 But the IDI would quickly find themselves on the receiving end of a tremendous amount of money for the full range of their activities. Sponsors and financial supporters over the years include:12, 13, 14, 15
- Abbott
- Aeras Global TB Vaccine Foundation
- Bill & Melinda Gates Foundation
- Centers for Disease Control and Prevention (CDC)
- Defense Threat Reduction Agency (DTRA)’s Cooperative Biological Engagement Program (CBEP)
- Doris Duke Charitable Foundation
- European Commission
- Foundation for Innovative New Diagnostics
- Gilead Sciences
- The Global Fund to Fight AIDS, Tuberculosis and Malaria
- Leidos
- Massachusetts General Hospital
- Merck
- Mérieux Foundation
- Microsoft Research
- National Institutes of Health (NIH)
- National Cancer Institute (NCI)
- National Institute of Allergy and Infectious Diseases
- Division of Acquired Immunodeficiency Syndrome (DAIDS)
- Novartis
- Pfizer
- President’s Emergency Plan for AIDS Relief (PEPFAR)
- President’s Malaria Initiative (PMI)
- RAND Corporation
- Rockefeller Foundation
- UNICEF
- Unitaid
- United States Agency for International Development (USAID)
- United States Army Medical Research Institute of Infectious Diseases (USAMRIID)
- United States Department of Defense
- University of Washington
- Wellcome Trust
- World Health Organization

Boulware’s focus during his time working with the Infectious Diseases Institute has been “understanding the pathogenesis of HIV immune reconstitution inflammatory syndrome (IRIS), an important complication of HIV therapy that has recently emerged with the roll out of antiretroviral therapy in Africa.” Specifically, he and his team have been “prospectively characterizing the differences in gene expression that occur over time among people who develop IRIS. The objective is to identify the pathophysiology of IRIS, identify biomarkers for the prediction and diagnosis of IRIS, and develop better treatments for and to prevent IRIS.”16
Boulware’s early interest in genetic medicine should not be overlooked. Neither should the fact that his colleagues at the time also took particular interest in mRNA as the mechanism for both cause of HIV-related disease and how to potentially treat it.

Dr. Bojnanen’s profile from January 2009 explains:17
“One mechanism that cells use to turn off gene expression is specific mRNA decay within the cytoplasm. Dr. Bohjanen is working to understand the biochemical mechanisms that regulate mRNA decay and to understand the role of mRNA decay in regulating gene expression in disease states such as malignancy or virus infection.”

Meanwhile, CIDMT Co-Director Mark Schleiss was “engaged in the study of CMV vaccines,” specifically (but not exclusively) “purified protein subunit and DNA vaccines.”18
Boulware’s various publications arising from this work disclose funding from the National Institute of Allergy and Infectious Diseases.19, 20 In 2009, he received a research grant from GlaxoSmithKline for a project titled “HIV Immune Reconstitution Inflammatory Syndrome in Uganda”, lasting until the end of 2015.21
From March 2015-February 2018, Boulware acted as Principal Investigator on a project at the Infectious Diseases Institute titled “Adjunctive Sertraline for the Treatment of HIV-Associated Cryptococcal Meningitis”.22 The resulting study was published in August 2019, and found that the Pfizer-developed antidepressant “did not reduce mortality and should not be used to treat patients with HIV-associated cryptococcal meningitis.”23 It also noted support from the Doris Duke Charitable Foundation, which — as I’ve previously noted — hosts former NIAID head Anthony Fauci and Gavi, the Vaccine Alliance director Afsaneh Beschloss on its board of trustees.24
Boulware began a second trial in May 2016 titled “Cryptococcal Antigen Screening Plus Sertraline (C-ASSERT)”.25 Originally scheduled to run until April 2023, the trial was cancelled in late 2019 after several Ugandan trial subjects suffered psychotic episodes associated with the sertraline, with one lasting greater than four months.26, 27

Note that sertraline (also known as Zoloft) is part of a class of drugs called selective serotonin reuptake inhibitors (SSRI) — a class which also includes a drug later explored for its effects on COVID-19, fluvoxamine.

While Boulware has spent years swimming in the pool of HIV/AIDS research, his real focus is on a condition called cryptococcal meningitis. In a 2016 article published in Newsweek, Boulware describes it as “so neglected that it’s not even considered a ‘neglected disease.’”28 “It just gets lumped with HIV, so no one sees it and no one really cares.” The next paragraph points out that so-called neglected diseases have “benefited significantly from product development partnerships” (PDP), for which the Bill & Melinda Gates Foundation provides the most funding globally, including to public-private partnerships like PATH, the Drugs for Neglected Diseases Initiative (DNDi), the TB Alliance. But somehow, Boulware’s niche disease wasn’t seeing any of that Gates funding. He advocates in particular for a new screening tool called the Cryptococcal Antigen Lateral Flow Assay, or CrAg LFA, developed by a diagnostics company called Immuno-Mycologics, or “IMMY”, which had received the support of the Clinton Health Access Initiative (CHAI).
The really interesting part of this article comes right at the end, where Newsweek reveals that it, too, had created the piece with funding from the Pulitzer Center on Crisis Reporting.

One of Boulware’s HIV studies at the IDI, published in December 2017, was featured in the Joint United Nations Programme on HIV/AIDS (UNAIDS)’ August 2018 report titled “Miles to Go”.29, 30, 31
Relationships with pharmaceutical companies

In a presentation delivered at the 2018 Mycoses Study Group Education & Research Consortium (MSGERC) biennial meeting, Boulware discloses that he has received research funding from none other than Gilead Sciences — the pharmaceutical company responsible for the development of the drug we now know as remdesivir.32
He also discloses serving as an advisor to Viamet and Minnetronix. Viamet Pharmaceuticals was a biotechnology company founded in 2005 by two researchers out of the University of North Carolina at Chapel Hill and Northwestern University.33 Viamet discovered and developed “small-molecule inhibitors of validated metalloenzymes via an innovative and proprietary metal-binding approach” called “Metallophile™ Technology.”34 Viamet’s investors included Astellas Venture Management, Eli Lilly’s Lilly Ventures and the Novartis Venture Fund.35
On June 3, 2015, Viamet announced it had been awarded a $1.95 million grant from the United States Department of Defense to develop “a novel topical antifungal agent to prevent and treat mold infections as a result of battlefield wounds” — more specifically, the treatment of Valley Fever.36 In April 2016, the company reported co-owning six patents with the United States government through the Department of Health and Human Services (HHS).37

Of particular interest to David Boulware would be Viamet’s drug called VT-1129, which it had developed for treatment of cryptococcal meningitis. Indeed, Boulware served as senior author on a study published in September 2016 that found “VT-1129 shows potential for use against fluconazole-resistant Cryptococcus,” for which he received funding from Viamet.38

Viamet was acquired by NovaQuest Capital management in January 2018 and renamed Mycovia Pharmaceuticals shortly after.39, 40
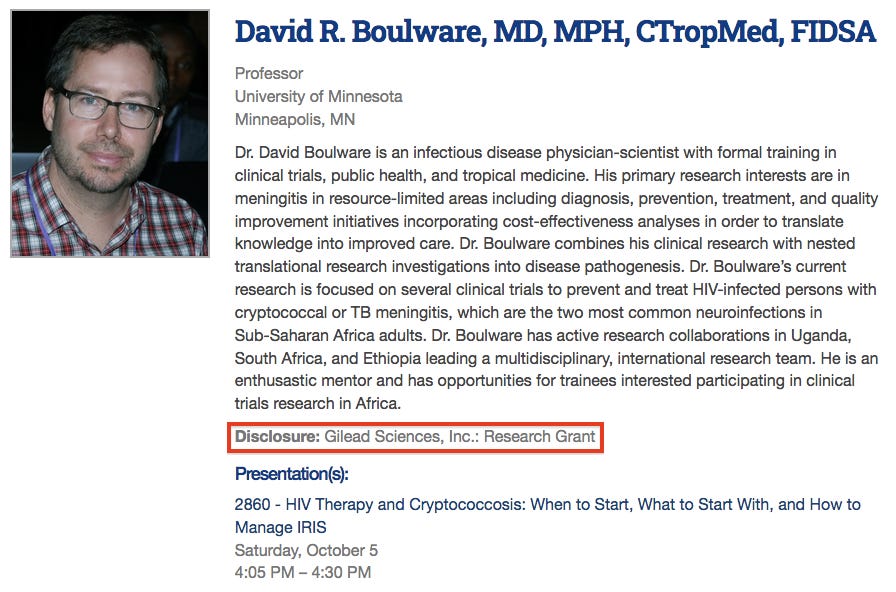
Boulware contributed as a site investigator for the AMBIsome Therapy Induction OptimisatioN clinical trial investigating the use of Gilead’s AmBisome in treating cryptococcal meningitis, published in the journal Trials in November 2018. The trial was sponsored by Gilead, who had also paid the senior author.41
Matinas BioPharma
Returning back to Boulware’s 2018 MSGERC presentation for a moment, we see that he is proposing running clinical trials on a drug called “oral amphotericin”, also to treat cryptococcal meningitis. Luckily for him, he would receive funding for this project from the National Institute of Neurological Disorders and Stroke (NINDS) in March 2019.42 Phase I of his clinical trial, titled “Encochleated Oral Amphotericin for Cryptococcal Meningitis Trial (EnACT)”, began at the Infectious Diseases Institute in Uganda on October 24, 2019.43

Amphotericin is an antifungal medication developed by a biopharmaceutical company called Matinas BioPharma. Matinas was founded in 2013 by Jerry Jabbour and Abdel Fawzy.44, 45, 46
On January 10, 2019, Matinas announced it had entered an agreement with an undisclosed pharmaceutical company to “evaluate synergistic effects of Matinas’ lipid-nano-crystal (“LNC”) platform delivery technology with their partner’s nucleic acid polymer technology.”47 CEO Jerome Jabbour said that the collaboration would “provide solutions for companies developing innovative nucleic acid polymers, small molecule drugs, vaccines, proteins and potentially even gene-editing technologies.”

What exactly is a “lipid-nano-crystal”? According to Matinas’ website, their “proprietary intracellular LNC platform is designed to safely deliver a broad range of potent medicines – including small molecules, drugs with blood level-limiting toxicities, nucleic acid polymers, proteins, peptides, vaccines, and gene editing technologies.”48 In other words, the LNC certainly seems very similar in premise and application to the now-familiar lipid nanoparticle (LNP), as used in the COVID-19 genetic vaccine products deployed by Pfizer-BioNTech and Moderna. The main difference, at least in terms of sales pitch, appears to be in their “natural, non-toxic and highly efficient spiral crystal… with the unique ability to be administered orally and through various other routes.”
The real shocker comes later down on the same webpage.

In December 2020, Matinas announced it had been tapped by the National Institute of Allergy and Infectious Diseases (NIAID) to evaluate oral formulations of Gilead Sciences’s antiviral drug remdesivir in its potential treatment against COVID-19.
Matinas’ good pandemic fortunes didn’t stop there. On April 11, 2022, Matinas and BioNTech announced a research collaboration “to evaluate a novel delivery technology for mRNA-based vaccines.”49
Then, in January 2023, Matinas and National Resilience “entered into a Material Transfer and Evaluation Agreement focused on exploring the potential for oral delivery of identified nucleic acids.”50
In early 2023, the company sought funding from the Biomedical Advanced Research and Development Authority (BARDA), and continued into its third project with Genentech. It’s unclear to me if Genentech was the mystery company first mentioned in 2019, or if that company remains unnamed.
While Boulware was not, to my knowledge, involved in any of the above research collaborations, they all occurred while he was actively running his EnACT clinical trial. Given that he has been on Matinas’ payroll since just prior to the declaration of the COVID-19 pandemic, the introduction of these new partnerships would begin to compound the perceived conflicts of interest for anything Boulware might touch related to COVID-19 moving forward. Of course, by 2019, he had already disclosed receiving research funding from Gilead.51
Note, of course, that all of the companies and organizations that Matinas describes are directly involved in the development, manufacturing or deployment of the various COVID-19 genetic vaccine products currently on the market. NIAID co-developed Moderna’s mRNA-1273 and owns the patented coronavirus spike protein used in both Moderna and Pfizer-BioTech’s products.52, 53 BioNTech needs no elaboration, as the primary developer of the BNT162b2 product marketed by Pfizer. National Resilience is the CIA/Bill & Melinda Gates Foundation-linked manufacturer of synthetic RNA in the United States and Canada, particularly for Moderna’s product, co-led by former FDA commissioner Scott Gotlieb.54 BARDA kickstarted Moderna with substantial early funding,55 and Genentech did the same with BioNTech.56
The EnACT study is marked as completed on ClinicalTrials.gov as of February 15, 2023.
COVID-19 early treatment

At the outset of the declared COVID-19 pandemic, David Boulware was quick to jump into action. In an NPR article published March 31, 2020, Boulware points out that his “normal research is doing clinical trials in Africa for fungal meningitis of the brain,” but on March 9, chose to get his team together and spin up some clinical trials for repurposed drugs to treat COVID-19.57
And thus, Boulware began his work as the principal investigator for the first randomized clinical trial testing hydroxychloroquine for its use against COVID-19. The trial, titled Post-exposure Prophylaxis or Preemptive Treatment for SARS-Coronavirus-2, kicked off on March 17, 2020.58

Funding for the trial was provided by Jan and David Baszucki, Steve Kirsch, the University of Minnesota Foundation, the Alliance of Minnesota Chinese Organizations and Minnesota Chinese Chamber of Commerce.59
David Baszucki is the creator of Roblox, a video game which saw “dramatic growth during the Covid-19 pandemic as children who were forced to stay home spent more time playing games.”60 It also has a bit of a “sex problem,” as noted in a report from BBC News outlining various instances of sexual content in the game ostensibly made for kids — including a so-called “virtual sexual assault.”61

Steve Kirsch is certainly no stranger to the Rounding the Earth audience. Steve founded the COVID-19 Early Treatment Fund (CETF) in April 2020 in order to fund trials on repurposed drugs, starting with Boulware’s trial on hydroxychloroquine.62 Boulware later told MIT Technology Review that “Kirsch was extremely helpful early on in the pandemic, stepping up to fund early treatment trials when the US government would not fund such studies.”63 Donors to the CETF included the Baszuckis, Elon Musk, Marc Benioff, Vint Cerf (co-inventor of the internet), the Skoll Foundation, and Vanguard Charitable.64

Despite the enthusiasm and name power of the trial funders, on June 3, 2020, Boulware’s team announced that they found that “hydroxychloroquine has no benefit over placebo in preventing COVID-19.”65
However, it is worth noting that the placebo in question was folic acid — a rather strange choice, given that folic acid had its own evidence of clinical benefit in helping patients with COVID-19.66, 67
A little-discussed point about this first trial was that it also had a Canadian branch, co-led by the University of Alberta, University of Manitoba and the Research Institute of the McGill University Health Centre.68 Further funding for the Canadian branch came from:
- Bridge to Health Medical and Dental
- Manitoba Medical Service Foundation
- McGill Interdisciplinary Initiative in Infection and Immunity (MI4)
- McGill University Health Centre (MUHC)
- Northern Alberta Clinical Trials and Research Centre
- Research Institute of St. Joe’s Hamilton
- St. Joseph’s Hospital Foundation
I’ve spent more time than most of my American friends researching the academic health networks here in Canada, as these were the institutions leading the charge in our COVID-19 response. One of the above funders that I find particularly interesting is the McGill Interdisciplinary Initiative in Infection and Immunity (MI4).
MI4 provided a significant amount of research funding towards projects related to COVID-19 through their “MI4 Emergency COVID-19 Research Funding” program. Here are some examples of titles of the funded projects:69
- Development of a new Virus-like Particle (VLP) vaccine against COVID-19
- Real-Time Tracking of COVID-19 Vaccine Development
- Ethical tensions of implementing research during a crisis: Understanding moral experiences of healthcare providers caring for patients who are enrolled in CoViD-19 clinical trials
- Expert and Lay Perceptions of Translation of COVID-19 Treatment and Vaccine Development
- Success Rate and Timeline for Development of Vaccines for Emerging and Reemerging Viral Infectious Diseases
- COVID-19 Vaccines – Novel Approaches to Tackle a Novel Disease
- Effect of coronavirus pandemic and health care system response on non-COVID-19-related mortality, post pandemic myocardial infarction and heart failure rates in Quebec; a collateral damage in this war?
- Health Communication, Sociocultural Diversity, and COVID-19
Notably, MI4 announced in December 2020 that it had received a $600,000 gift from Pfizer towards the creation of the Pfizer Early Career Investigator Awards.70
In June 2020, Gilead Sciences announced that their newly-Emergency Use Authorized remdesivir would come at a cost of $3,120 per dose. Asked for comment, Boulware offered that “from the health system perspective, if remdesivir can shorten duration of hospitalization by four days, then the medicine provides a reasonable value.”71
TOGETHER Trial

Hydroxychloroquine wasn’t the end of the line for David Boulware. He also participated as a senior investigator in the TOGETHER Trial, a multinational clinical trial platform launched by researchers in Canada and Brazil.72
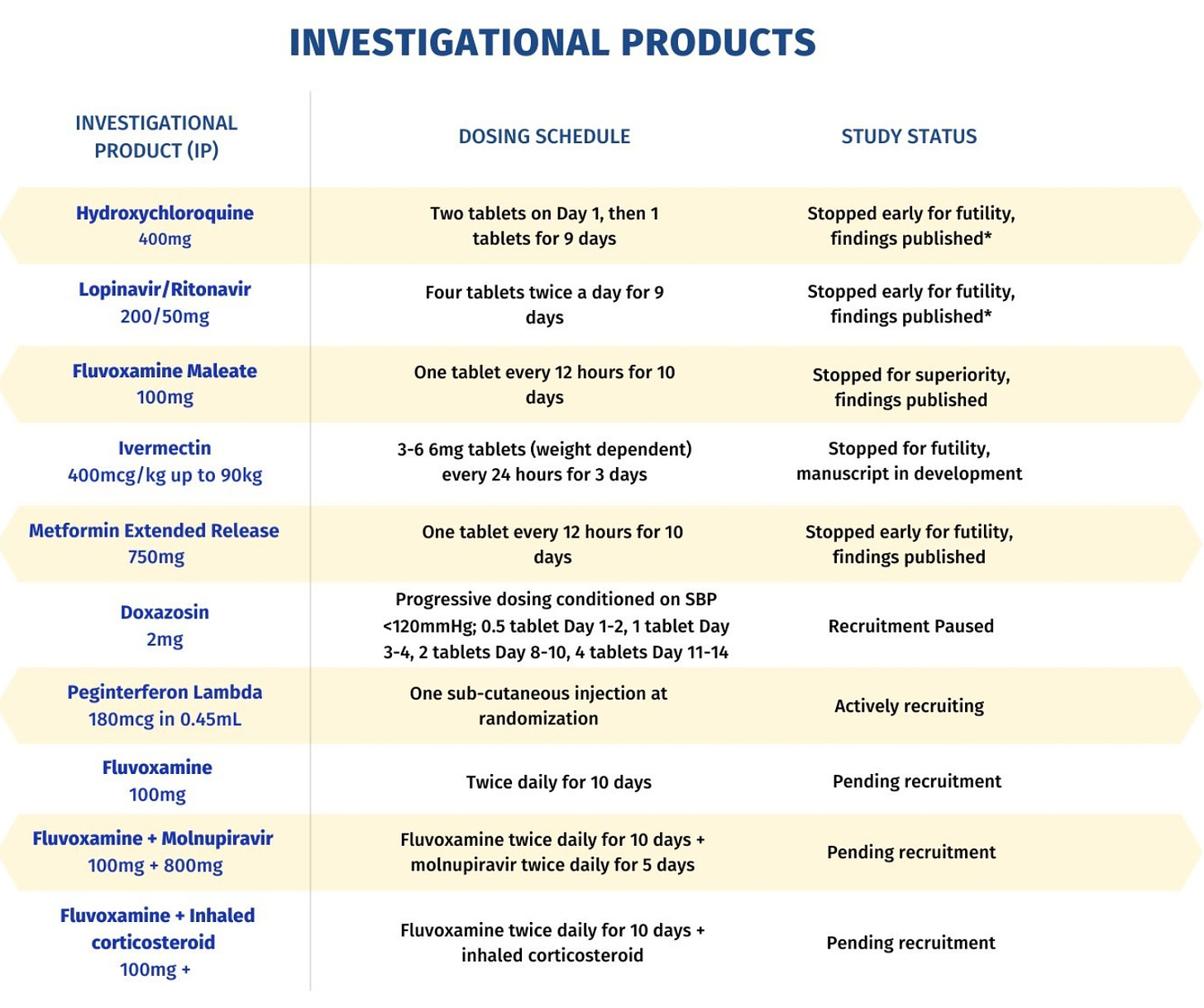
True to its name, the TOGETHER Trial really brought everything together under one roof. In addition to its own hydroxychloroquine trial which ran in Brazil,73 the international investigators also tested HIV drugs lopinavir and ritonavir, the antidepressant fluvoxamine, molnupiravir, ivermectin, inhaled corticosteroids, metformin, interferon lambda and doxazosin.74
Boulware participated as an investigator in the ivermectin arm, which “concluded that ivermectin is not useful against the disease.”75 The New York Times quoted Boulware in their coverage of the study as saying “There’s really no sign of any benefit. Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin towards other therapies.”76
The TOGETHER Trial was funded by the likes of the FTX Foundation, as well as Elon Musk, the Chan Zuckerberg Initiative (CZI), Twitter co-founder Jack Dorsey, LinkedIn co-founder Reed Hoffman, tech entrepreneur Yuri Milner, Google co-founder Eric Schmidt’s Schmidt Ventures and others through Fast Grants.77
ACTIV-6

Finally, Dr. Boulware currently serves as the national co-chair of the trial steering committee for the National Institutes of Health’s ACTIV-6 platform clinical trial.78
ACTIV-6 is part of the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiative launched on April 17, 2020.79 In its own words, ACTIV is a public-private partnership whose executive leadership includes/included Anthony Fauci, Peter Marks and Janet Woodcock, along with top representatives from Pfizer, BARDA, and the FDA, among other pharmaceutical companies whose treatments and tests were deployed during the COVID-19 response.80
Its pre-clinical working group includes Ralph Baric from the University of North Carolina at Chapel Hill, and is stock full of representatives from Pfizer, the Bill & Melinda Gates Foundation, Gilead Sciences, Merck, Sanofi, GlaxoSmithKline, the United States Army Medical Research Institute of Infectious Diseases, and Janssen.81
Conclusion
In looking over the career of David Boulware, I see a familiar theme: a man of passion and ambition working towards a successful career endpoint. For Boulware, cryptococcal meningitis is what he has always come back to, including during his work on COVID-19 early treatment trials. But just like many others who found themselves in positions of influence since 2020, his own ambition will always assert itself, and thus, it’s understandable why he would make decisions that favour the advancement of his own research.
In this case, recall that Boulware’s work from 2006 onwards has been experimenting on sick people in Uganda under the banner of the HIV/AIDS pandemic, just like Anthony Fauci, Rochelle Walensky, Margaret Hamburg and many others who have sat in the seat of power. There’s a nice way to put that, and a realistic way to put that. Realistically, the facility where he worked — the Infectious Diseases Institute — was quite literally co-founded by a Pfizer CEO, and funded heavily by Gilead Sciences, Johnson & Johnson, the Bill & Melinda Gates Foundation, and various branches of the United States government. Those include Fauci’s National Institute of Allergy and Infectious Diseases (NIAID), Walensky’s Centers for Disease Control and Prevention (CDC), Hamburg’s Food and Drug Administration (FDA), and even Robert Malone’s colleagues at the Department of Defense’s Defense Threat Reduction Agency (DTRA).
Both in the IDI and at home, Boulware’s research funding came from NIAID, from DoD-funded pharmaceutical companies, and from Gilead Sciences itself. The co-directors of his research institution have long focused on genetic aspects of viral infection, and naturally, the genetic medicines to combat it.
It seems to me that through the perceived failure of repurposed drugs to manage the COVID-19 “pandemic,” a wave of new technologies in genetic medicine have now been rolled out to the global population and normalized under the misleading banner of “vaccination”. Boulware’s most recent funder, Matinas BioPharma, has benefited tremendously from this new reality.
While this is simply an exploration and not a firm conclusion, it’s hard to see how David Boulware didn’t walk out of this as a man further along in his career, in large part thanks to his role in quashing hydroxychloroquine, ivermectin, et al — rather, and more importantly, the notion that early COVID-19 treatment existed at all. In the presence of such treatment possibilities, companies like Matinas, National Resilience, BioNTech, Gilead Sciences and Moderna would all be in a different place right now.
And if there’s any question as to where Dr. Boulware lands on these genetic medicines, we need only turn to his own words.
There’s this tweet from September 17, 2022:82

And this hot take following news that Moderna’s flu “vaccine” didn’t work:83

Moderna Inc (MRNA.O) on Thursday said its closely watched experimental messenger RNA-based influenza vaccine generated a strong immune response against A strains of the flu but failed to show it was at least as effective as an approved vaccine versus less prevalent influenza B.
The results dashed investor hopes that the company might plug its COVID franchise decline, sending Moderna’s shares down more than 6% in after-hours trading.
Dr. David Boulware, an infectious disease specialist at the University of Minnesota Medical School, said he was not overly concerned about the immune response versus Influenza B.
Boulware said the immune response against the A strains demonstrated that the vaccine probably worked and Moderna’s tweaks to the vaccine are likely to improve the response against the B strains.
“I consider it pretty positive,” he said.
Needless to say, Dr. Boulware, I hope you’re right about everything. About the uselessness of the most promising early treatment protocols, about the safety and efficacy of the various COVID-19 vaccine products… about it all.
Sadly, I don’t think you are.
References
- Paige, R. (2020, April 2). With a Gift for Science, Boulware ’96 Seeks Cure. Wabash College. http://archive.today/2023.05.17-233333/https://www.wabash.edu/news/story/11580 ↩︎
- Dr. David Boulware ’96/King Distinguished Professor at UMN – Alumni News. (2011, November 4). Wabash College. http://archive.today/2023.05.17-050444/https://blog.wabash.edu/alumninews/2011/11/04/dr-david-boulware-96king-distinguished-professor-at-umn/ ↩︎
- Boulware, D. R. (2021). Clinical & Translational Research in Cryptococcosis. Mycoses Study Group Education & Research Consortium. https://web.archive.org/web/20230517231334/https://msgerc.org/resources/Documents/Boulware%20Slides_4.26.pdf ↩︎
- Home – MED – DOM. Infect Diseases Microbiol Trans Research, University of Minnesota. Retrieved June 9, 2010, from https://web.archive.org/web/20100609211421/http://www.med.umn.edu/cidmtr/ ↩︎
- Schmickle, S. (2008, November 13). University of Minnesota doctors battle AIDS in Uganda. MinnPost. http://archive.today/2010.06.23-113510/http://www.minnpost.com/stories/2008/11/13/4537/university_of_minnesota_doctors_battle_aids_in_uganda ↩︎
- Donors. Pulitzer Center. Retrieved May 29, 2022, from http://archive.today/2022.05.29-181734/https://pulitzercenter.org/about/donors ↩︎
- Ronald, A., Kamya, M., Katabira, E., Scheld, W. M., & Sewankambo, N. (2011). The Infectious Diseases Institute at Makerere University, Kampala, Uganda. Infectious Disease Clinics of North America, 25(2), 369–383. https://doi.org/10.1016/j.idc.2011.02.007 ↩︎
- Background. Infectious Diseases Institute. Retrieved May 17, 2023, from http://archive.today/2023.05.17-071738/https://idi.mak.ac.ug/who-we-are/about/ ↩︎
- Startup Programs. Pangaea Global AIDS Foundation. Retrieved August 11, 2002, from http://archive.today/2002.08.11-080145/http://www.pgaf.org/programs.html ↩︎
- Annual Report 2012. Infectious Diseases Institute. Retrieved May 17, 2023, from https://web.archive.org/web/20230517220105/https://idi.mak.ac.ug/wp-content/uploads/2017/08/IDI-Annual-Report-2012.pdf ↩︎
- Van Houten, P. (2011, June 22). Tibotec Therapeutics Becomes Janssen Therapeutics, Part Of The Janssen Pharmaceutical Companies. Johnson & Johnson. http://archive.today/2023.05.17-061755/https://www.jnj.com/media-center/press-releases/tibotec-therapeutics-becomes-janssen-therapeutics-part-of-the-janssen-pharmaceutical-companies ↩︎
- Annual Report July 2014 – June 2015. Infectious Diseases Institute. Retrieved July 6, 2017, from https://web.archive.org/web/20170706002552/https://idi-makerere.com/index.php?option=com_docman&task=doc_download&gid=94&Itemid=565 ↩︎
- Annual Report 2013. Infectious Diseases Institute. Retrieved July 6, 2017, from https://web.archive.org/web/20170706003837/https://idi-makerere.com/index.php?option=com_docman&task=doc_download&gid=17&Itemid=565 ↩︎
- Annual Report 2012. Infectious Diseases Institute. Retrieved May 17, 2023, from https://web.archive.org/web/20230517220105/https://idi.mak.ac.ug/wp-content/uploads/2017/08/IDI-Annual-Report-2012.pdf ↩︎
- Research Projects. (2017, August). Infectious Diseases Institute. https://web.archive.org/web/20230517200932/https://idi.mak.ac.ug/wp-content/uploads/2017/08/Research-Projects.pdf ↩︎
- David R. Boulware, M.D., MPH, DTM&H; – MED – DOM – Infect Diseases Microbiol Trans Research, University of Minnesota. Center for Infectious Diseases and Microbiology Translational Research. Retrieved June 21, 2010, from https://web.archive.org/web/20100621123616/http://www.cidmtr.umn.edu/investigators/boulware/home.html ↩︎
- Paul Bohjanen, M.D., Ph. D. – MED – DOM. (2009, January 30). Infect Diseases Microbiol Trans Research, University of Minnesota. https://web.archive.org/web/20100613154723/http://www.med.umn.edu/cidmtr/investigators/bohjanen/home.html ↩︎
- Mark R. Schleiss, M.D. – MED – DOM. (2009, January 11). Infect Diseases Microbiol Trans Research, University of Minnesota. https://web.archive.org/web/20100613161306/http://www.med.umn.edu/cidmtr/investigators/schleiss/home.html ↩︎
- Kambugu, A., Meya, David B., Rhein, J., O’Brien, M., Janoff, Edward N., Ronald, Allan R., Kamya, Moses R., Mayanja‐Kizza, H., Sande, Merle A., Bohjanen, Paul R., & Boulware, David R. (2008). Outcomes of Cryptococcal Meningitis in Uganda Before and After the Availability of Highly Active Antiretroviral Therapy. Clinical Infectious Diseases, 46(11), 1694–1701. https://doi.org/10.1086/587667 ↩︎
- Boulware, D. R., Callens, S., & Pahwa, S. (2008). Pediatric HIV immune reconstitution inflammatory syndrome. Current Opinion in HIV and AIDS, 3(4), 461–467. https://doi.org/10.1097/coh.0b013e3282fe9693 ↩︎
- HIV Immune Reconstitution Inflammatory Syndrome in Ugand. Experts@Minnesota. Retrieved May 18, 2023, from https://web.archive.org/web/20230518195434/https://experts.umn.edu/en/projects/hiv-immune-reconstitution-inflammatory-syndrome-in-ugand ↩︎
- Adjunctive Sertraline for the Treatment of HIV-Associate. Experts@Minnesota. Retrieved May 18, 2023, from http://archive.today/2023.05.18-200104/https://experts.umn.edu/en/projects/adjunctive-sertraline-for-the-treatment-of-hiv-associate ↩︎
- Rhein, J., Huppler Hullsiek, K., Tugume, L., Nuwagira, E., Mpoza, E., Evans, E., Kiggundu, R., Pastick, K. A., Ssebambulidde, K., Akampurira, A., Williams, D. A., Bangdiwala, A. S., Abassi, M., Musubire, A. K., Nicol, M. R., Muzoora, C., Meya, D. B., Boulware, D. R., Ndyetukira, J. F., & Ahimbisibwe, C. (2019). Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infectious Diseases, 19(8), 843–851. https://doi.org/10.1016/s1473-3099(19)30127-6 ↩︎
- Board of Trustees. Doris Duke Charitable Foundation. Retrieved June 26, 2022, from http://archive.today/2022.06.26-144104/https://www.ddcf.org/who-we-are/our-people/board-of-trustees/ ↩︎
- Cryptococcal Antigen Screening plus Sertraline (C-ASSERT). Experts@Minnesota. Retrieved May 18, 2023, from https://web.archive.org/web/20230518204336/https://experts.umn.edu/en/projects/cryptococcal-antigen-screening-plus-sertraline-c-assert ↩︎
- University of Minnesota, Infectious Diseases Institute, Uganda, National Institute of Allergy and Infectious Diseases (NIAID), & Mbarara University of Science and Technology. (2020, June 2). Cryptococcal Antigen Screening Plus Sertraline. ClinicalTrials.gov. https://web.archive.org/web/20230518204446/https://clinicaltrials.gov/ct2/show/results/NCT03002012 ↩︎
- Boulware, D. R., Nalintya, E., Rajasingham, R., Kirumira, P., Naluyima, R., Turya, F., Namanda, S., Rutakingirwa, M. K., Skipper, C. P., Nikweri, Y., Hullsiek, K. H., Bangdiwala, A. S., & Meya, D. B. (2020). Adjunctive sertraline for asymptomatic cryptococcal antigenemia: A randomized clinical trial. Medical Mycology, 58(8), 1037–1043. https://doi.org/10.1093/mmy/myaa033 ↩︎
- Adams, P. (2016, January 4). Crushing Crypto, a Deadly, Neglected Disease. Newsweek. https://web.archive.org/web/20230518201802/https://www.newsweek.com/crushing-crypto-world-health-organization-neglected-disease-411193 ↩︎
- UNAIDS. (2018). Miles to Go—closing gaps, breaking barriers, righting injustices (p. 103). Joint United Nations Programme on HIV/AIDS. https://web.archive.org/web/20230508052828/https://www.unaids.org/sites/default/files/media_asset/miles-to-go_en.pdf ↩︎
- Flynn, A., Godwin Anguzu, Mubiru, F., Kiragga, A., Kamya, M. R., Meya, D. B., Boulware, D. R., Kambugu, A., & Castelnuovo, B. (2017). Socioeconomic position and ten-year survival and virologic outcomes in a Ugandan HIV cohort receiving antiretroviral therapy. PLOS ONE, 12(12), e0189055–e0189055. https://doi.org/10.1371/journal.pone.0189055 ↩︎
- IDI research manuscript cited in UNAIDS 2018 global report. Infectious Diseases Institute. Retrieved December 8, 2022, from https://web.archive.org/web/20221208223013/https://idi.mak.ac.ug/idi-research-manuscript/ ↩︎
- MSGERC Headquarters. (2018, October 25). MSGERC 2018: Cryptococcus: David Boulware. YouTube. https://youtu.be/QnQ1k4YcCWM ↩︎
- Viamet Pharmaceuticals – Crunchbase Company Profile & Funding. Crunchbase. Retrieved May 18, 2023, from https://www.crunchbase.com/organization/viamet-pharmaceuticals ↩︎
- Our Company. Viamet Pharmaceuticals. Retrieved January 3, 2009, from http://archive.today/2009.01.03-114852/http://www.viamet.com/viamet.asp?id=269&category=2 ↩︎
- Viamet Pharmaceuticals – Funding, Financials, Valuation & Investors. Crunchbase. Retrieved May 18, 2023, from https://www.crunchbase.com/organization/viamet-pharmaceuticals/company_financials ↩︎
- Katz, R. (2015, June 3). Viamet Announces Awarding Of Department Of Defense Grant. BioSpace. http://archive.today/2023.05.18-215031/https://www.biospace.com/article/releases/viamet-announces-awarding-of-b-department-of-defense-b-grant-/?s=80 ↩︎
- Viamet Pharmaceutical Holdings, LLC. (2016, April 8). Form S-1. Securities and Exchange Commission. http://archive.today/2023.05.18-215855/https://www.sec.gov/Archives/edgar/data/1538928/000119312516535590/d117508ds1.htm%23rom117508_10 ↩︎
- Nielsen, K., Vedula, P., Smith, K. C., Meya, D. B., Garvey, E. P., Hoekstra, W. G., Schotzinger, R. J., & Boulware, D. R. (2016). Activity of VT-1129 againstCryptococcus neoformansclinical isolates with high fluconazole MICs. Medical Mycology, 55(4), myw089–myw089. https://doi.org/10.1093/mmy/myw089 ↩︎
- Tuck, S., & MacDougall Biomedical Communications. (2018, January 3). NovaQuest Capital Management to Acquire Viamet Pharmaceuticals and the VT-1161 Antifungal Program. Bloomberg. http://archive.today/2023.05.18-213244/https://www.bloomberg.com/press-releases/2018-01-03/novaquest-capital-management-to-acquire-viamet-pharmaceuticals-and-the-vt-1161-antifungal-program ↩︎
- Teater, B. (2022, April 29). Durham startup Mycovia wins FDA approval for new antifungal drug. WRAL TechWire. https://web.archive.org/web/20230518213439/https://wraltechwire.com/2022/04/29/durham-startup-mycovia-wins-fda-approval-for-new-antifungal-drug/ ↩︎
- Lawrence, D. S., Youssouf, N., Molloy, S. F., Alanio, A., Alufandika, M., Boulware, D. R., Boyer-Chammard, T., Chen, T., Dromer, F., Hlupeni, A., Hope, W., Hosseinipour, M. C., Kanyama, C., Lortholary, O., Loyse, A., Meya, D. B., Mosepele, M., Muzoora, C., Mwandumba, H. C., & Ndhlovu, C. E. (2018). AMBIsome Therapy Induction OptimisatioN (AMBITION): High Dose AmBisome for Cryptococcal Meningitis Induction Therapy in sub-Saharan Africa: Study Protocol for a Phase 3 Randomised Controlled Non-Inferiority Trial. Trials, 19(1). https://doi.org/10.1186/s13063-018-3026-4 ↩︎
- Encochleated Oral Amphotericin for Cryptococcal Meningitis Trial. Experts@Minnesota. Retrieved May 9, 2023, from https://web.archive.org/web/20230509022530/https://experts.umn.edu/en/projects/encochleated-oral-amphotericin-for-cryptococcal-meningitis-trial ↩︎
- Matinas BioPharma Nanotechnologies, Inc., & University of Minnesota. (2023, March 29). Encochleated Oral Amphotericin for Cryptococcal Meningitis Trial. ClinicalTrials.gov. https://web.archive.org/web/20221026222118/http://clinicaltrials.gov/ct2/show/nct04031833 ↩︎
- Matinas BioPharma Holdings, Inc. Annual report pursuant to Section 13 and 15(d). (2014). Matinas BioPharma. http://archive.today/2023.05.18-224839/https://www.matinasbiopharma.com/investors/sec-filings/all-sec-filings/xbrl_doc_only/1114 ↩︎
- Jerry Jabbour, Matinas BioPharma Holdings inc: Profile and Biography. Bloomberg. Retrieved May 18, 2023, from http://archive.today/2023.05.18-225130/https://www.bloomberg.com/profile/person/18631011 ↩︎
- Abdel A. Fawzy, Ph.d. | Matinas Biopharma | United States of America. OMICS International. Retrieved May 18, 2023, from http://archive.today/2023.05.18-225547/https://biography.omicsonline.org/united-states-of-america/matinas-biopharma/abdel-a-fawzy-phd-1369145 ↩︎
- Matinas BioPharma Holdings. (2019, January 10). Matinas BioPharma Announces a Research Evaluation with Top Global Pharma Company Based on Its Proprietary Drug Delivery Platform. GlobeNewswire News Room. https://web.archive.org/web/20230509042135/https://www.globenewswire.com/news-release/2019/01/10/1686096/32419/en/Matinas-BioPharma-Announces-a-Research-Evaluation-with-Top-Global-Pharma-Company-Based-on-Its-Proprietary-Drug-Delivery-Platform.html ↩︎
- LNC Platform. Matinas BioPharma Holdings, Inc. Retrieved May 9, 2023, from http://archive.today/2023.05.09-041901/https://www.matinasbiopharma.com/lnc-technology/lnc-platform ↩︎
- Zacks Equity Research. (2022, April 12). Matinas (MTNB) Up on Deal With BioNTech for mRNA Vaccines. Nasdaq. https://web.archive.org/web/20220412202120/https://www.nasdaq.com/articles/matinas-mtnb-up-on-deal-with-biontech-for-mrna-vaccines ↩︎
- Bhargava, A. (2023, January 23). Matinas BioPharma Provides Business Update and 2023 Strategic Outlook. Matinas BioPharma Holdings, Inc. http://archive.today/2023.01.31-021839/https://www.matinasbiopharma.com/investors/news-events/press-releases/detail/439/matinas-biopharma-provides-business-update-and-2023 ↩︎
- David R. Boulware, MD, MPH, CTropMed, FIDSA. IDWeek 2019. Retrieved May 18, 2023, from http://archive.today/2023.05.18-221228/https://www.eventscribe.com/2019/IDWeek/fsPopup.asp?Mode=presenterInfo&PresenterID=688680 ↩︎
- Rizvi, Z. (2020, June 25). The NIH Vaccine. Public Citizen. http://archive.today/2021.01.01-104304/https://www.citizen.org/article/the-nih-vaccine/ ↩︎
- COVID-19 Vaccines: Dr. Fauci’s Team May Personally Profit. (2020, October 11). ICAN – Informed Consent Action Network. http://archive.today/2023.03.01-015445/https://icandecide.org/article/covid-19-vaccines-dr-faucis-team-may-personally-profit/ ↩︎
- Webb, W. (2022, August 17). RNA for Moderna’s Omicron Booster Manufactured by CIA-Linked Company. Unlimited Hangout. http://archive.today/2022.08.23-051250/https://unlimitedhangout.com/2022/08/investigative-reports/rna-for-modernas-omicron-booster-manufactured-by-cia-linked-company/ ↩︎
- Moderna, Inc. (2018). Form S1 (p. 186). U.S. Securities and Exchange Commission. http://archive.today/2022.02.20-165037/https://www.sec.gov/Archives/edgar/data/1682852/000119312518323562/d577473ds1.htm ↩︎
- BioNTech AG. (2016, September 21). BioNTech to enter into worldwide strategic collaboration with Genentech to develop individualized mRNA cancer therapies. Cision PR Newswire. https://www.prnewswire.com/news-releases/biontech-to-enter-into-worldwide-strategic-collaboration-with-genentech-to-develop-individualized-mrna-cancer-therapies-594231481.html ↩︎
- Palca, J. (2020, March 31). Clinical Trials Set To Determine If Anti-Malaria Drug Effective Against COVID-19. NPR. https://web.archive.org/web/20230509021308/https://www.npr.org/sections/coronavirus-live-updates/2020/03/31/824572119/clinical-trials-set-to-determine-if-anti-malaria-drug-effective-against-covid-19 ↩︎
- Home | Post-exposure Prophylaxis or Preemptive Treatment for Coronavirus. (2020, June 3). University of Minnesota. http://archive.today/2020.07.18-030252/https://covidpep.umn.edu/ ↩︎
- Support | Post-exposure Prophylaxis or Preemptive Treatment for Coronavirus. University of Minnesota. Retrieved December 11, 2022, from http://archive.today/2022.12.11-173505/https://covidpep.umn.edu/support ↩︎
- Game maker Roblox’s value rockets seven-fold during pandemic. (2021, January 7). BBC News. https://web.archive.org/web/20221016232651/http://www.bbc.com/news/business-55570009 ↩︎
- Clayton, J., & Dyer, J. (2022, February 15). Roblox: The children’s game with a sex problem. BBC News. http://archive.today/2022.02.15-090031/https://www.bbc.com/news/technology-60314572 ↩︎
- Saey, T. H. (2020, August 24). New treatments aim to treat COVID-19 early, before it gets serious. Science News. http://archive.today/2021.10.13-001342/https://www.sciencenews.org/article/coronavirus-covid-19-new-early-treatments ↩︎
- Ferguson, C. (2021, October 5). This tech millionaire went from covid trial funder to misinformation superspreader. MIT Technology Review. http://archive.today/2022.11.26-095935/https://www.technologyreview.com/2021/10/05/1036408/silicon-valley-millionaire-steve-kirsch-covid-vaccine-misinformation/ ↩︎
- Donors. COVID-19 Early Treatment Fund. Retrieved September 29, 2021, from http://archive.today/2021.09.29-172054/https://www.treatearly.org/donors ↩︎
- Boulware, D. R., Pullen, M. F., Bangdiwala, A. S., Pastick, K. A., Lofgren, S. M., Okafor, E. C., Skipper, C. P., Nascene, A. A., Nicol, M. R., Abassi, M., Engen, N. W., Cheng, M. P., LaBar, D., Lother, S. A., MacKenzie, L. J., Drobot, G., Marten, N., Zarychanski, R., Kelly, L. E., & Schwartz, I. S. (2020). A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. New England Journal of Medicine, 383(6). https://doi.org/10.1056/nejmoa2016638 ↩︎
- Doidge, N. (2020, August 14). Hydroxychloroquine: A Morality Tale. Tablet Magazine. http://archive.today/2021.08.23-011706/https://www.tabletmag.com/sections/science/articles/hydroxychloroquine-morality-tale ↩︎
- Acosta-Elias, J., & Espinosa-Tanguma, R. (2020). The Folate Concentration and/or Folic Acid Metabolites in Plasma as Factor for COVID-19 Infection. Frontiers in Pharmacology, 11. https://doi.org/10.3389/fphar.2020.01062 ↩︎
- University of Minnesota, McGill University Health Centre/Research Institute of the McGill University Health Centre, University of Manitoba, & University of Alberta. (2021, May 13). Post-exposure Prophylaxis or Preemptive Therapy for SARS-Coronavirus-2: A Pragmatic Randomized Clinical Trial. ClinicalTrials.gov. https://web.archive.org/web/20230513212649/https://www.clinicaltrials.gov/ct2/show/NCT04308668 ↩︎
- Results of MI4 Emergency COVID-19 Research Funding. MI4. Retrieved May 16, 2023, from https://web.archive.org/web/20230516234033/https://www.mcgill.ca/mi4/mi4-supported-research/emergency-covid-19-research-funding ↩︎
- Clement, J., & Schwartz, T. (2020, December 4). Pfizer Canada donation will help new researchers change the future. McGill University Faculty of Medicine and Health Sciences. http://archive.today/2022.08.23-174216/https://www.mcgill.ca/medhealthsci/channels/news/pfizer-canada-donation-will-help-new-researchers-change-future-326710 ↩︎
- Drug company to charge thousands for coronavirus treatment. (2020, June 29). NBC News; Associated Press. http://archive.today/2020.06.29-144326/https://www.nbcnews.com/health/health-news/remdesivir-coronavirus-gilead-charge-thousands-treatment-u-s-n1232385 ↩︎
- Together Trial. Platform Life Sciences. Retrieved November 15, 2022, from http://archive.today/2022.11.15-224049/https://platformlifesciences.com/together-trial/ ↩︎
- Reis, G., Moreira Silva, E. A. dos S., Medeiros Silva, D. C., Thabane, L., Singh, G., Park, J. J. H., Forrest, J. I., Harari, O., Quirino dos Santos, C. V., Guimarães de Almeida, A. P. F., Figueiredo Neto, A. D. de, Savassi, L. C. M., Milagres, A. C., Teixeira, M. M., Simplicio, M. I. C., Ribeiro, L. B., Oliveira, R., & Mills, E. J. (2021). Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19. JAMA Network Open, 4(4), e216468. https://doi.org/10.1001/jamanetworkopen.2021.6468 ↩︎
- Trial specifications. TOGETHER Trial. Retrieved November 14, 2022, from http://archive.today/2022.11.14-223307/https://www.togethertrial.com/trial-specifications ↩︎
- Hooper, C. L., & Henderson, D. R. (2022). Ivermectin and the TOGETHER Trial. Cato Institute. http://archive.today/2023.01.24-133325/https://www.cato.org/regulation/summer-2022/ivermectin-together-trial ↩︎
- Zimmer, C. (2022, March 30). Ivermectin Does Not Reduce Risk of Covid Hospitalization, Large Study Finds. The New York Times. http://archive.today/2022.03.31-172826/https://www.nytimes.com/2022/03/30/health/covid-ivermectin-hospitalization.html ↩︎
- Home. Fast Grants. Retrieved December 23, 2021, from http://archive.today/2021.12.23-005332/https://fastgrants.org/ ↩︎
- Naggie, S., Boulware, D. R., Lindsell, C. J., Stewart, T. G., Slandzicki, A. J., Lim, S. C., Cohen, J., Kavtaradze, D., Amon, A. P., Gabriel, A., Gentile, N., Felker, G. M., Jayaweera, D., McCarthy, M. W., Sulkowski, M., Rothman, R. L., Wilson, S., DeLong, A., Remaly, A., & Wilder, R. (2023b). Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial. JAMA. https://doi.org/10.1001/jama.2023.1650 ↩︎
- ACTIV. National Institutes of Health (NIH). Retrieved April 1, 2022, from http://archive.today/2022.01.11-042823/https://www.nih.gov/research-training/medical-research-initiatives/activ ↩︎
- ACTIV Public-Private Partnership. (2022). National Institutes of Health. https://web.archive.org/web/20220402051949/https://www.nih.gov/sites/default/files/research-training/initiatives/activ/activ-org-chart_3.2.22.pdf ↩︎
- Preclinical Working Group. (2022, March 8). National Institutes of Health (NIH). http://archive.today/2022.04.02-062343/https://www.nih.gov/research-training/medical-research-initiatives/activ/preclinical-working-group ↩︎
- David Boulware, MD MPH [@boulware_dr]. (2022, September 17). “This sub-study of the @umnmedschool Covid-Out trial demonstrates that having had a #covid19 vaccine booster prior is the best early treatment. Nothing else reduces symptoms or resolves symptoms faster, even if at low risk of hospitalization. Get boosted! #IDtwitter @ashishkjha” [Tweet]. Twitter. https://web.archive.org/web/20230519182039/https://twitter.com/boulware_dr/status/1571114481991946252 ↩︎
- Wingrove, P., & Erman, M. (2023, February 17). Moderna flu vaccine delivers mixed results in trial, shares fall. Reuters. https://web.archive.org/web/20230519182240/https://www.reuters.com/business/healthcare-pharmaceuticals/moderna-flu-vaccine-delivers-mixed-results-trial-2023-02-16/ ↩︎

