Originally published February 13, 2023, on Substack
Introduction
Over the last two years, there has been much discussion about the possibility that the Government of Canada, or possibly Prime Minister Justin Trudeau himself, holds a financial stake in the COVID-19 mRNA-based products deployed across the country.
If this is true, this is concerning on its face; if either Trudeau, his foundation, or the government he leads stood to profit from this specific pharmaceutical countermeasure, that would constitute a significant conflict of interest. Depending on the specifics of the arrangement, this could be seen by the Canadian public as yet another in a line of political corruption scandals under Trudeau’s belt. Alternatively, the actions taken by the government under the banner of the COVID-19 public health response could suggest a much more serious crime of racketeering.
Most of the discussion surrounding this topic has been confused, incomplete or misguided. Indeed, it looks like there have been literal “fake news” stories injected into this realm of discussion in order to distract those who are earnest, but easily taken by shiny objects. This extends even to the dedicated doctors, lawyers and concerned citizens working behind the scenes to decipher the facts of the situation in order to pursue legal action. I know, because I’m part of no less than three teams doing just that.
It is my opinion that this needs to be properly documented and understood in order to move action forward in the court of public opinion and the court of law. Today, I will attempt to do just that.

This article was born out of a need to correct the record in the Canadian context, but wound up growing to encompass the developmental history of the products as a whole. As such, I hope this article winds up being informative for my friends all around the world — after all, we’re all in this together.
The Tech: Gene Transfer, LNPs and modRNA
As with anything important during the COVID-19 era, this story requires a basic knowledge of the developmental history of the three key pieces of underlying technology in the mRNA shots. They are inextricably linked, as both the lipid nanoparticles and the modified RNA were specifically employed for their ability to help bypass the natural immune system of the human body when experimenting with gene transfer. Examining the partnerships and licensing agreements made to combine these three unique technologies also allows us to take a look into who created what, and who now profits from the associated patents.
Gene transfer
Jumping ahead just a tad, let’s quickly define what constitutes a lipid nanoparticle vs. a liposome.
Lipid nanoparticles (LNP) are tiny, artificial balls of fat that are designed to carry fragile drugs, including RNA or DNA payloads, into the human recipient.1 They are a step above previously-existing materials called liposomes, which are less sophisticated hollow balls that are more prone to breaking apart.2
Liposomes for gene transfer
Liposomes were first used to successfully deliver a drug in the human body in the 1970s.3 In the late 1980s, Dr. Robert Malone discovered a method to use liposomes to cause RNA sequences to transfect into human, mouse and rat cells.4

After successfully delivering an mRNA sequence into frog embryos in 1989, Malone began to express his excitement about the potential of using the liposome/mRNA technology for highly-targeted medical treatments.5 He continued his work at a biotechnology company called Vical, where he and his collaborators at the University of Wisconsin demonstrated that they could spur protein production in live mice using the mRNA and lipid combination.6 This led to the registration of several patents in 1989 bearing Malone’s name, specifically referencing the ability to spur an immune response using this tech.7, 8, 9
Manufacturing challenges
Malone left Vical due to a number of internal disputes, after which the company’s patented gene therapy technology was licensed by Merck for the purpose of developing mRNA and DNA vaccines.10 Merck attempted to replicate the successes in-house, but were unable to address issues in manufacturing complexity.

In 1993, French biotech company Transgène had their own successes with mRNA,11 following similar outcomes the year prior by the Scripps Research Institute in reversing diabetes in rats.12 Still, due to high costs and uncertain sustainability, both Merck and Transgène opted to focus on DNA and viral vector vaccines instead.13
Modified RNA
As the focus moved away from vaccines, the field of oncology began early trials to use mRNA technology to reduce cancerous tumours.14

In 1996, researcher Eli Gilboa and his team at Duke University Medical Center demonstrated the use of immune cells combined with tumour genes to kickstart the body’s own anti-cancer response.15 This inspired the creation of CureVac in 2000 to begin their own experiments with the technology in hopes of commercializing it.16, 17 Unfortunately, despite being the first mRNA therapeutic company, CureVac would fail to show the safety or efficacy of its attempts.18
Pseudouridine

A significant roadblock in making these genetic products work was the mRNA itself, as it degraded too quickly in practice. This is in part because of the body’s own ability to quickly get rid of foreign genetic material, a problem encountered by biochemist Katalin Karikó as she attempted to improve upon Malone’s work to prove mRNA’s potential as a drug platform.19, 20
Using funding from the National Institutes of Health,21 she and her colleague Drew Weissman discovered an alteration that could be made to synthetic mRNA’s code that would allow it to bypass the patient’s immune response by using an artificial “pseudo-nucleotide” to replace uracil (one of the four “building blocks” of RNA).22, 23

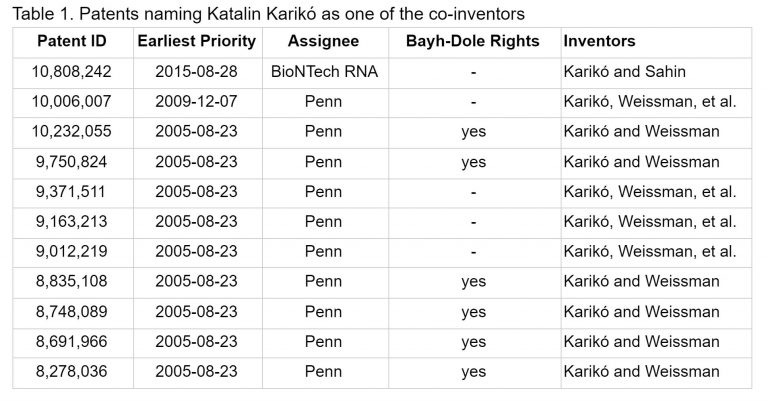
Various patents for this method were filed, with ownership shared across the scientists, University of Pennsylvania, and the United States Government, as a result of the Bayh-Dole Act (a set of provisions allowing the government to claim a non-exclusive license to use the invention it helped fund).24, 25 UPenn sold an exclusive license for some of the patents to a company called CellScript, making it difficult for Karikó and Weissman to use their own invention.26 Karikó would go on to become Senior Vice President at BioNTech, a biotechnology company who also aggressively pursued the development of mRNA vaccines in the decade to come.27
Other researchers began using this method, which also showed promise in guiding the function of cells while helping the injected mRNA avoid winding up integrating into the recipient’s genome – an unprecedented risk of accidental gene editing.28 One such researcher was Derrick Rossi, a stem-cell biologist at Boston Children’s Hospital in Massachusetts. Shortly following his successful use of Karikó’s pseudouridine in his own lab, he co-founded the company now known as Moderna, appropriately named based on a portmanteau of “modified RNA”.29
But there remained one key piece required to complete the puzzle.
Lipid nanoparticles
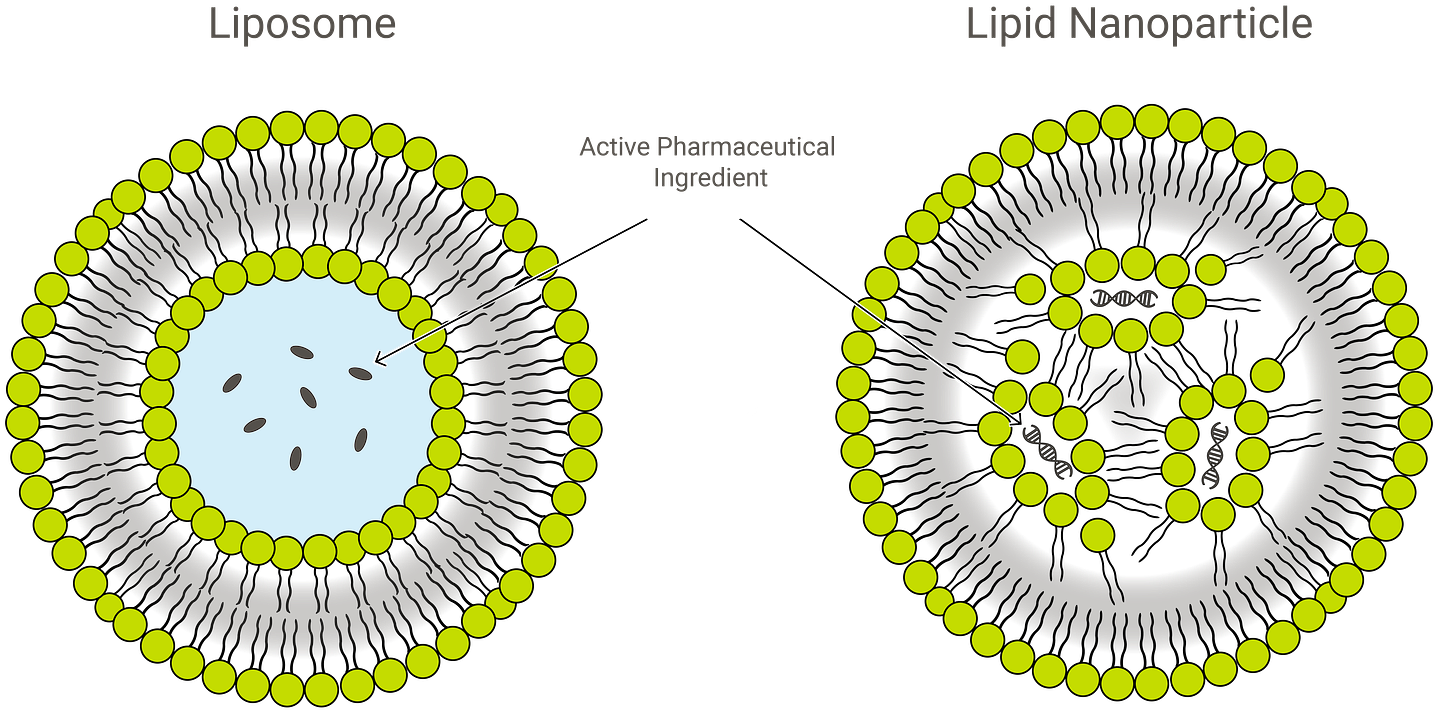
The second piece of novel technology required to make mRNA gene-transfer “vaccines” work is the lipid nanoparticle. As already described above, lipid nanoparticles (LNP) are tiny, artificial balls of fat that are designed to carry fragile drugs, including RNA or DNA payloads, into the human recipient.
Inex Pharmaceuticals

Dr. Robert Malone credits the biggest accomplishment in this space to Dr. Pieter Cullis of the University of British Columbia.30 Dr. Cullis was among the scientists experimenting with liposomes in the early 1980s, fascinated by their properties in many aspects of human biology.31 He discovered he could load cancer drugs into liposomes, inspiring him and several colleagues to form Inex Pharmaceuticals in 1992.32

To improve prospects of successfully advancing gene therapy techniques, Cullis developed and patented a new form of lipid nanoparticle designed to deliver genetic material to host cells.33 From 1994 onward, a series of patents were filed describing the technology Cullis and his colleagues created, including the addition of a chemical called polyethylene glycol (PEG) to further increase the LNP’s ability to pass into cells.34, 35, 36, 37
Protiva Biotherapeutics
This included a variation on the LNP design that Cullis developed in 2000 with several colleagues including Dr. Ian MacLachlan, which became the basis for a spinoff company the team named Protiva Biotherapeutics, focused entirely on gene therapy.38, 39 The company received a $14.5 million investment from Lumira Ventures in 2001,40 and further investments in 2002 from BDC Venture Capital.41

Protiva partnered with Roche subsidiary Alnylam Pharmaceuticals in Cambridge, Massachusetts to collaboratively develop products using RNAi to inhibit, or “turn off”, target genes (compared to mRNA, which turns on production of proteins), which bore fruit in a 2006 study demonstrating success silencing genes in monkeys.42
Tekmira Pharmaceuticals
In 2001, Inex had made deals to develop a product with GlaxoSmithKline.43 By 2004, Inex was in the research stage of preparing their own targeted “cancer vaccine” program.44 However, after the FDA declined to approve their chemotherapy products, Cullis quit, the company downsized and rebranded to Tekmira Pharmaceuticals.45, 46 This led to $28.5 million in funding from “large biotechnology companies” for further development of anti-cancer drugs and RNA-based treatments for other diseases.47

Protiva raised a total of $8.3 million across two funding rounds in July 2006 and July 2007, comprised of investments from the Canadian Medical Discoveries Fund, Kinetic Capital, BDC Venture Capital and GrowthWorks Capital.48
In 2008, Tekmira and Protiva reunited their intellectual property and signed a new deal with Alnylam Pharmaceuticals to allow access to their lipid nanoparticle patents – a key component of achieving their self- described “Alnylam 2020” strategy.49, 50
Acuitas Therapeutics

Also in 2008, Cullis received a $100,000 grant from the Canadian Institutes of Health Research (CIHR) via the University of British Columbia to continue improvements on the durability of the lipid nanoparticle.51 In February 2009, Cullis and longtime collaborators Drs. Thomas Madden and Michael Hope founded AlCana Technologies, which was later renamed Acuitas Therapeutics.52
With so many patents, inventors and companies now in play, management of interests and ownership became challenging. An agreement was signed on July 27, 2009, mutually establishing the rights and limitations held by Alnylam, UBC, AlCana/Acuitas, Tekmira and Protiva, while explicitly acknowledging the complex nature of the variety of relationships they all had with each other.53
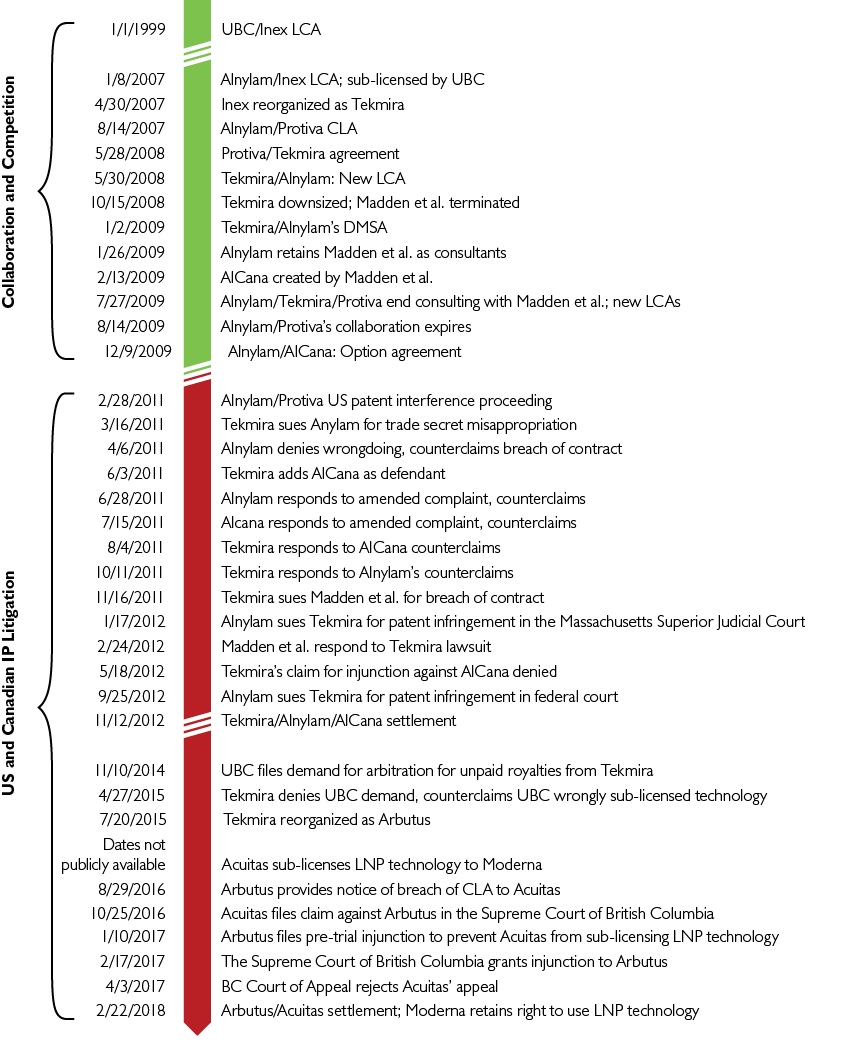
In June 2011, Tekmira filed a lawsuit against both Alnylam and AlCana/Acuitas, seeking damages for (among other things) “misappropriation of trade secrets, tortious interference with contractual relations, unjust enrichment, unfair and deceptive acts and trade practices, and civil conspiracy.”54
Canadian Government Funding
Meanwhile, the following month, AlCana/Acuitas received the first of several funding packages from the Canadian government through the National Research Council Canada (NRC).55

As the government later boasted, AlCana/Acuitas was incubated with “over 5 years of advisory and R&D funding support” through the NRC’s Industrial Research Assistance Program (IRAP) through which the company was “able to establish their internal chemistry program, hire new PhD expertise to their team, and develop its LNP delivery system for mRNA.”56 Further multi-year grants were issued in 2012, 2013 and 2015.57, 58, 59, 60 Cullis himself continued to receive CIHR research grants for LNP development in 2010, 2012, 2015, 2020 and 2022.61 In April 2012, Protiva Biotherapeutics received their own $150,000 grant from NRC.62
However, it turns out the Government of Canada was providing financial support for the development of the lipid nanoparticles much earlier than 2011.

A quarterly report filed with the Securities and Exchange Commission (SEC) dated September 2015 reveals that on November 12, 1999, Inex Pharmaceutics “entered into a Technology Partnerships Canada (“TPC”) agreement with the Canadian Federal Government” to support the development of “certain oligonucleotide product candidates”.63
According to ScienceDirect, oligonucleotides are small sequences of RNA or DNA that are used in a wide variety of applications — in particular, the suppression or activation of genes through target genetic therapy.64, 65
The case between Tekmira and Alnylam was settled in November 2012, resulting in a restructured licensing agreement allowing Alnylam to resume using Tekmira’s LNP patents under terms with new “clarity.”66 This also allowed Acuitas to acquire a sublicense for some of the LNP technology from Tekmira – and conversely, Tekmira to use Acuitas’ RNAi tech – an arrangement which went into effect in December 2013.67
Arbutus Biopharma
The United States Department of Defense awarded Tekmira a $140 million contract to develop a treatment against Ebola in response to the 2013 outbreak in West Africa, but the product failed to pass safety and efficacy testing and was pulled from trials in 2015.68, 69 The company merged with OnCore Biopharma, an offshoot of Gilead Sciences.70 This led to yet another name change to Arbutus Biopharma.

In June 2016, Arbutus filed a rather alarming-sounding patent titled “Delivering CRISPR therapeutics with lipid nanoparticles”.71 The patent was published in April 2020.
In October 2016, Alnylam was forced to cancel development of their mRNA-based therapy for genetic heart disease after a clinical trial showed it may have been killing participants.72
In April 2018, Arbutus announced that they were joining forces with the Swiss biotech company Roivant Sciences to launch Genevant Sciences, a jointly-owned venture focused on “the discovery, development, and commercialization of a broad range of RNA-based therapeutics” aiming for “5-10 product candidates into the clinic by 2020.”73
In October 2019, Arbutus was forced to pull yet another drug from trial after their Hepatitis B treatment caused recipients to develop the disease.74
The Companies: Moderna and BioNTech
Once the trifecta was complete, with patented gene transfer technology augmented and expanded through the combination of patented modified RNA encapsulated in patented lipid nanoparticles, the stage was set to go big in the biotech startup space.
Moderna
As was briefly touched on earlier, a stem cell biologist named Derrick Rossi achieved his own success experimenting with modified RNA in his lab at Boston Children’s Hospital. This resulted in an April 2010 patent application and subsequent publication describing “a simple, nonintegrating strategy for reprogramming cell fate based on administration of synthetic mRNA modified to overcome innate antiviral responses.”75 Rossi’s patent explicitly describes “methods, compositions, and kits comprising synthetic, modified RNAs” which “can be used either to express a desired protein in a cell or tissue, or to change the differentiated phenotype of a cell to that of another, desired cell type.”76
Simply put, this was the technology to cause the human body to change itself at the genetic level.
Enter the Venture Capital firms

Rossi’s results with the technique pioneered by Karikó attracted interest from bioengineer Dr. Robert Langer of the Massachusetts Institute of Technology (MIT) and Noubar Afeyan, CEO of a Cambridge venture capital firm called Flagship Pioneering (formerly Flagship Ventures). Coming off of a series of investments in other promising startups (including Selecta Biosciences, a company developing a platform to reduce unwanted immune responses),77 the team of investors in and around Flagship saw the commercial potential of the pseudouridine-modified RNA platform.78

Together, along with Harvard cardiovascular biologist Dr. Kenneth Chien, the group founded ModeRNA Therapeutics in September 2010.79, 80
In addition to the seed funding from Flagship, the company received an undisclosed amount of funding from a shadow investor to kickstart their new innovations with modified RNA and their ambitions with stem cell technology.81 In March 2013, Moderna received their first research grant from the Defense Advanced Research Programs Agency (DARPA) — the research arm of the U.S. Department of Defense — in an award titled “[m]odified RNA technology for production of 14 antibodies for immune prophylaxis.”82

Since its founding, Moderna has attempted to bring gene therapy products to market but has consistently failed to develop a therapy safe enough to administer in human clinical trials. This included an mRNA therapy for heart disease developed in collaboration with AstraZeneca,83 and a series of rare disease vaccines with Alexion.84, 85 Broadly speaking, the company kept its operations “mysterious” and under wraps, with very little in the way of published materials beyond patent filings.86
Moderna had, however, managed to develop lucrative partnerships with the world’s vaccine and oncology giants based on the theory that they could get their platform to work – including breaking fundraising records for the pharmaceutical industry in 2015.87 That same year, the Bill & Melinda Gates Foundation invested $52 million into CureVac’s future attempts at mRNA vaccines.88 They then turned their attention to Moderna, entering “a global health project framework agreement” with up to $100 million in funding for an experimental HIV mRNA vaccine.89 In June 2016, Moderna signed a $200 million deal with Merck to develop cancer immunotherapy products at a new manufacturing facility.90

In September 2016, Moderna received their first funding contract from the Biomedical Advanced Research and Development Authority (BARDA), the arm of the U.S. Department of Health and Human Services responsible for developing “medical countermeasures” including vaccines. The agency awarded Moderna $125 million to develop an mRNA vaccine for the Zika virus.91
Moderna needed access to the lipid nanoparticle delivery system, for which they had sought and received four sub-licenses from Acuitas in 2016.92 This led to legal action from Arbutus, striking the previous contract between the two Vancouver companies and preventing Acuitas from issuing any further licenses.93 A court decision later granted Arbutus full ownership over the licenses in question, which the company described as the consolidation of “its LNP intellectual property estate.”94 Moderna was, however, cleared to continue using the LNP tech granted under the existing four sub-licenses within Canada.95, 96

While Moderna had attempted to create its own version of pseudouridine, the company eventually signed a non-exclusive licensing agreement with CellScript (the patent holder for Karikó’s invention) in June 2017.97 Karikó’s own employer BioNTech found themselves in the same dilemma, signing a similar agreement with CellScript on July 14, 2017.98
In November 2017, Moderna’s chief medical officer Tal Zaks delivered a TED Talk titled “The disease-eradicating potential of gene editing”.99
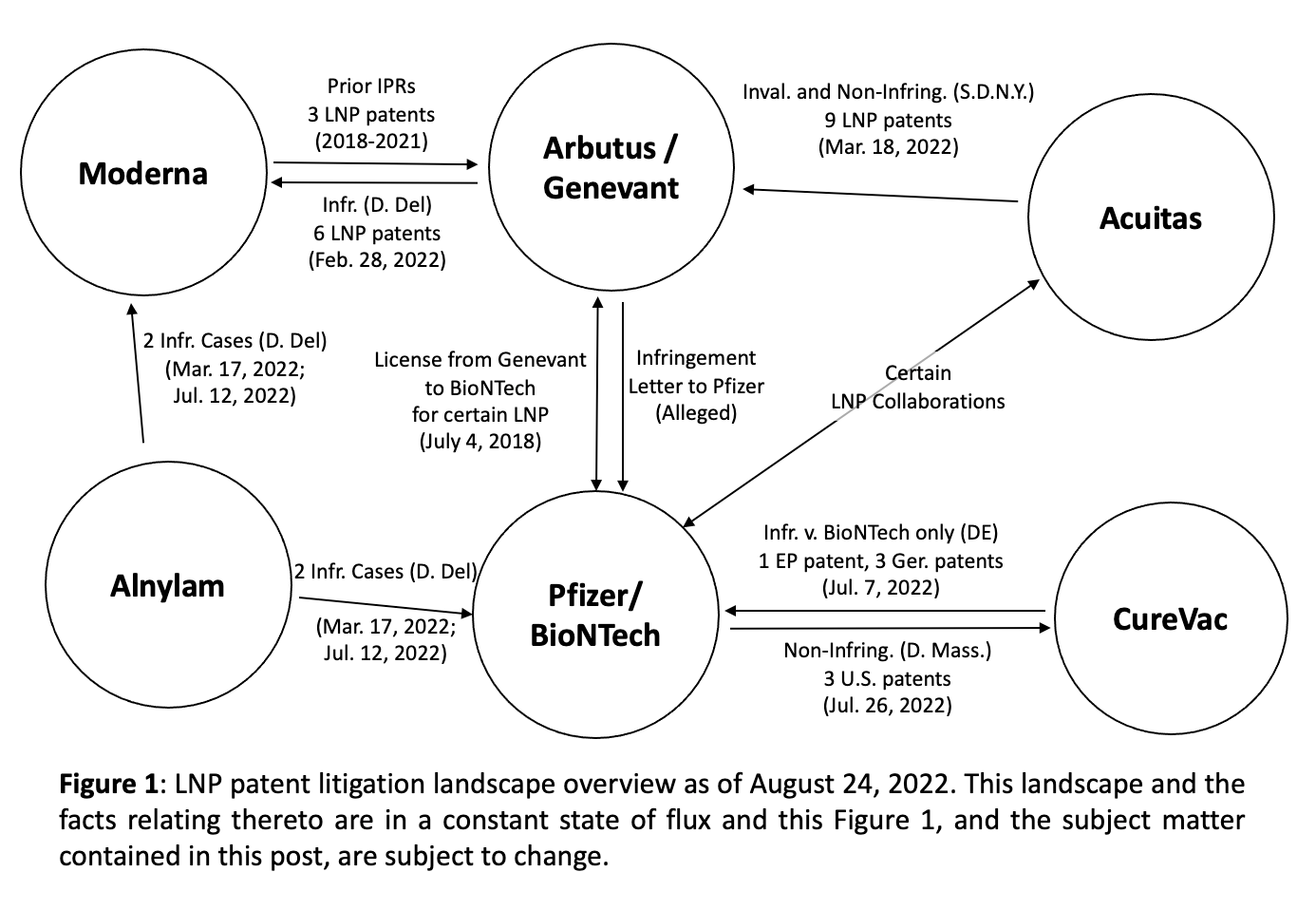
In February and March 2018, Moderna asked a court to review two Arbutus patents on the grounds that they shouldn’t be patentable in the first place.100 Litigation on several more patents continued through to 2022 with both sides (and their partners) suing back and forth, and the outcome is yet to be decided definitively.101, 102 Despite this, Moderna was in control of the two key pieces of the mRNA vaccine puzzle as 2019 drew to a close: the modified RNA, and the lipid nanoparticle.
BioNTech

BioNTech was founded in 2008 by Ugur Sahin, Christoph Huber and Özlem Türeci with a seed investment of €150 million from the Strüngmann family, through its investment vehicle AT Impf, and MIG Capital.103
As previously described, Katalin Karikó joined BioNTech as a senior vice president in 2013, having successfully invented the means by which her new employer would eventually succeed with gene therapy.
In 2015 and 2016, BioNTech was given a large financial boost by several pharmaceutical companies including Eli Lilly, Genmab, Bayer, Genentech and Sanofi.104, 105, 106 The pharmaceutical giants were eager to capitalize on the promising market of personalized cancer vaccines, which BioNTech’s mRNA platform was attempting to manifest.107 Their work was published in the scientific literature, marking their slow progress towards a viable product.108
On July 10, 2018, BioNTech announced it had entered an agreement with Genevant Sciences to collaborate on mRNA vaccine candidates using Genevant’s lipid nanoparticle technology on exclusive license. With this deal, BioNTech gained access to the lynchpin LNP patents controlled by Arbutus Biopharma through Genevant, which it had formed with Roivant Sciences just months prior. BioNTech signed on for Genevant’s highly convenient year of 2020 as the target for clinical trials.109 The plan included using RNA interference (RNAi), mRNA, and gene editing as the mechanisms for their products.
On August 16, 2018, BioNTech announced they had entered a partnership with Pfizer to develop an mRNA-based flu vaccine.110 Kathrin Jansen, Senior Vice President and Head of Pfizer’s Vaccine Research and Development Unit explained that beyond the seasonal flu, the mRNA technology was required in order to “respond rapidly and in quantity to pandemic influenza threats.” She also noted mRNA vaccines’ ability “to code for any protein or multiple proteins.” The deal put Pfizer in the driver’s seat, giving it full responsibility for development and marketing of the vaccines after the first successful human study.
Sanofi returned with another large investment in January 2019.111 This was followed in September by a $55 million buy-in from the Bill & Melinda Gates Foundation, with an option to increase the amount to $100 million.112 As with their investment in Moderna, the deal was centred around Gates’ interest in the development of HIV vaccines, as well as for tuberculosis. On October 16, 2019, Nature published an article describing BioNTech’s successes in testing experimental immunotherapy vaccines for cancer, and suggested the progress could be applied to solve problems like rabies and pandemic influenza.113
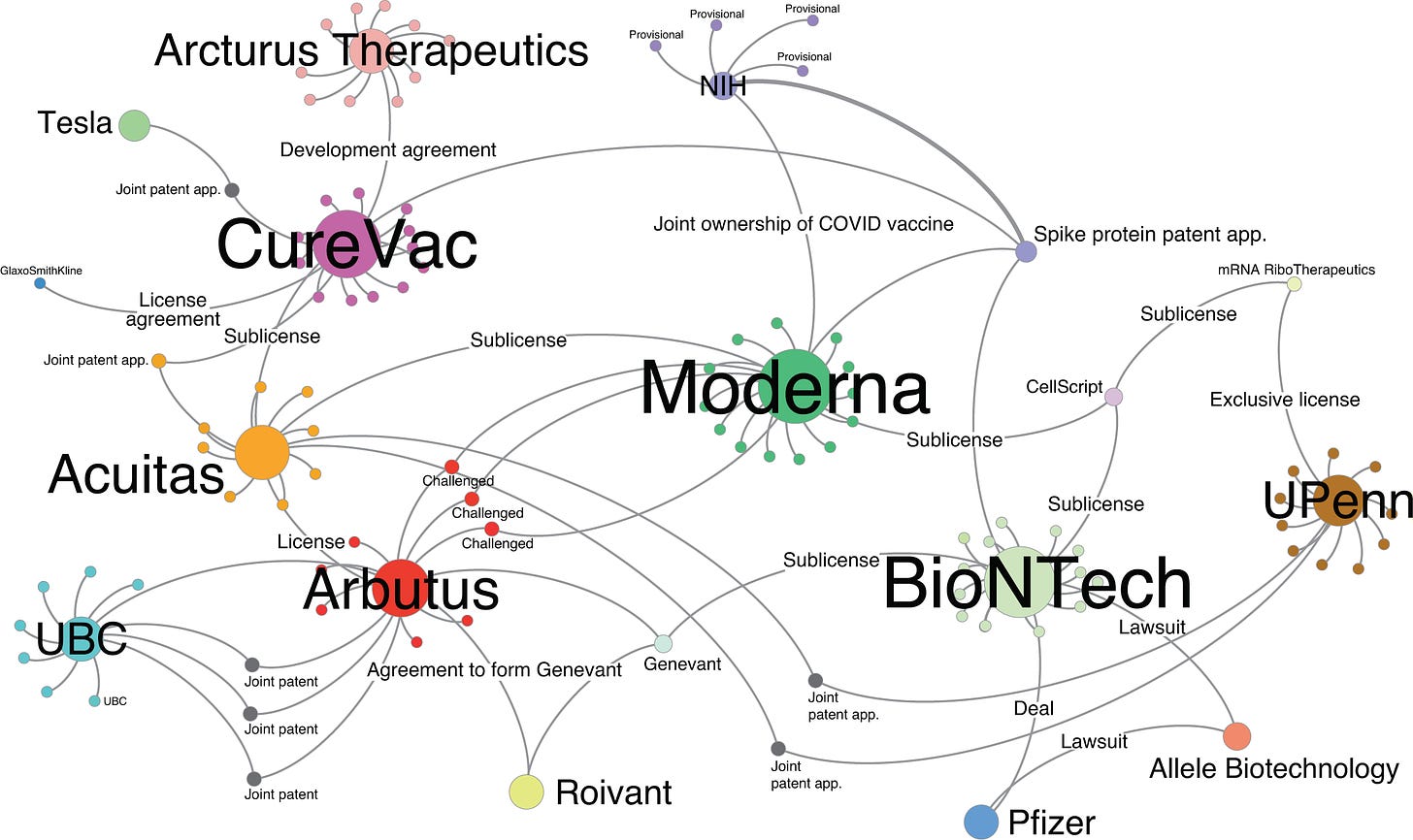
As with Moderna, BioNTech’s series of lucrative co-development deals and angel investments equipped the company with both the pseudouridine and lipid nanoparticle licenses required to position themselves perfectly for the upcoming “race” to respond to a supposedly novel viral outbreak.
The result was two mRNA COVID-19 vaccine products: Pfizer-BioNTech’s BNT162b2 and Moderna’s mRNA-1273.
COVID-19 kicks off in Canada – with a speech
On March 11, 2020, the World Health Organization declared COVID-19 to be a “Public Health Emergency of International Concern” (PHEIC).114 Over the following week, provinces and municipalities across the country instituted various forms of public health emergencies, and the federal government closed its borders.115
Then, on April 9, 2020, Prime Minister Justin Trudeau delivered the speech that Dr. David Martin cites as the nail in the coffin.116 Watch the below video starting at 15:46:
“Our healthcare systems across the country are coping — for the time being. But we’re at a fork in the road between the best and the worst possible outcomes.
The best possible outcome is no easy path for any of us. The initial peak, the top of the curve, may be in late spring with the end of the first wave in the summer. As Dr. [Teresa] Tam explained, there will likely be smaller outbreaks for a number of months after that.
This will be the new normal… until a vaccine is developed.
But, as we saw, that is so much better than we could face, all of us, if we do not rise to the challenge of this generation. The path we take is up to us. It depends on what each of us does right now. It will take months of continued, determined effort. We’ll need to keep practicing physical distancing, staying home, and washing our hands. It will help. It will help get the numbers that Dr. Tam was talking about, between 4,000 and 44,000 deaths, as low as possible.”
– Justin Trudeau
Addressing the Claims
Now that we’ve covered the history of the lipid nanoparticle technology and its role in the advancement of gene therapy as a product class, let’s address the specific claims being made about Justin Trudeau and Canada’s possible wrongdoing.
David Martin

In an August 2021 discussion with Vaccine Choice Canada, Dr. David Martin told Ted Kuntz and crew that the Government of Canada played a key role in the creation of the mRNA COVID-19 injections. Here is a transcript of exactly what Dr. Martin said, edited down to include only the relevant parts.117
“The University of British Columbia, working in partnership with Inex Pharmaceuticals in 2005, developed the lipid nanoparticle technology that ultimately became the basis of the formation of a company in British Columbia called Tekmira Pharmaceuticals.
But the problem was they ran out of funding. So there was a series of reorganizations, and in those reorganizations, two companies were formed: Arbutus [Biopharma] and Acuitas [Therapeutics]. Acuitas was the one that, unfortunately, the Government of Canada has not told the citizens of Canada, is the reason why both Moderna and Pfizer have the ability to deliver the current bioweapons program.
And I think most people would be shocked to find out that when you have the Prime Minister of Canada getting up in front of a camera in the spring of 2020, telling the world that the only way forward is to allegedly return to a “new normal” when there is a vaccine… What Trudeau did not tell the public was that he had a financial stake in the outcome of that being the selected pathway forward. What he didn’t tell the Canadian public was that Canada’s blight on the moral record of what has been historically an amazingly wonderful set of innovations coming out of the Canadian research institutions and research laboratories in fact created the mechanism whereby you could take mRNA and inject it into a population and try to stabilize that injection.
The lipid nanoparticle technology that was developed, and ultimately passed to Arbutus, was the subject of a licensing agreement that was made with Acuitas [Therapeutics] in British Columbia — a private company, who conveniently had very little reporting requirements. And Acuitas misappropriated the lipid nanoparticle technology and ultimately made it available to both BioNTech and Moderna.
It is absolutely critical for us to understand that without the Canadian contribution of the lipid nanoparticle technology from British Columbia, we would have no meaningful response in the form of what’s being called a vaccination, and we would not have a bioweapons program. That’s a pretty important statement to make to an audience largely of Canadians, and it would be very interesting to find out why it is that Trudeau has not admitted to the public and has been unwilling to actually put into the public record the what we know to be at least billions of dollars of concessions.
We know that in the case of Pfizer-BioNTech, that last quarter alone, somewhere between eight and 9 billion came in the form of the revenue off of all of the interventions that are being sold off as coronavirus vaccines.
One person actually owned this company, Thomas Madden, who’s the CEO of Acuitus. In 2009, he was largely the sole owner of it.
He actually appropriated the technology in a labor dispute, which functionally was a trade secret argument around this. …in 2016, somebody in Canada knew that there was something going to happen with this particular vaccine platform because in 2016, Arbutus [Biopharma] and Acuitas [Therapeutics] got into litigation on whether or not the license for the lipid nanoparticle technology that Acuitas had from Arbutus was in fact capable of being extended to other pathogens.
And in 2016, there was a significant amount of litigation and the license that Acuitas had to use lipid nanoparticle technology developed by Tekmira, developed by Arbutus… The license was actually terminated in 2016. That coincides with the weaponization of SARS-CoV-2. Now, do we have at this moment in time the written record of the evidence of what we know was knowable?
The answer to that is no. There is no public information that currently exists that has been made in any format that any of us can access. There’s no public information to tell us what precisely transpired in 2016, which allowed this particular dispute to erupt between these two Canadian firms, all based in the history of Tekmira.
But somewhere in 2016, somebody knew that there was a lottery win to be had. And my guess is that somewhere inside of the Canadian health system, and somewhere inside of NIAID in the Vaccine Research Center, and somewhere inside the [University of North Carolina at Chapel Hill] records, we will find that the Trudeau government was fully aware by at least 2018 that we were going to have a significant pandemic requiring this core technology to be unleashed on the world, courtesy of the Canadian collaboration on lipid nanoparticles.
And there is no question that by the time we get to 2019, March, specifically of 2019, we know that Arbutus, Moderna, Pfizer, BioNTech, and others, were in fact working on a vaccine for a respiratory pathogen, and we know that information because they amended their patent filings to say exactly that.“
– David E. Martin
It is worth noting that the following message is displayed early on in the video, declining to formally endorse anything Dr. Martin said as anything beyond hearsay.

And that is wise of them! There’s a lot of interesting tidbits here, but within the confines of the video itself, no specific documents are referenced or provided.
All of that said, it appears that Dr. Martin’s story is large accurate, except in the areas where he expressly speculates. Within the confines of this video, Dr. Martin does not suggest that Justin Trudeau owns any shares of any of the relevant companies. I believe that Dr. Martin missed the mark on communicating his point clearly, which is that the lipid nanoparticle patents in question were developed in partnership and with the funding of the Canadian government — not simply within Canada’s borders, which is the impression the video leaves one with.

He made more pointed claims in a November 2021 speech, stating that “the Canadian government gets a kickback for every one of the Pfizer and Moderna shots” because of Acuitas’ and Arbutus’ licensing agreements with the vaccine makers. He provides no further evidence to support the claim, simply repeating his broad outline from before.
I want to make clear, however, that Dr. Martin carved the path through the stone that allowed further investigation to occur. I do not want to come across as overly critical, especially given the countless hours he surely spent arriving to the place he did. And he’s been very clear that more research — and action — was still required.
Robert Malone
While Martin didn’t say it, Malone did.
In Canada, there is speculation that Justin Trudeau and his family's foundation holds 40% of Acuitas, which is a manufacturer of mechanic lipids used by Pfizer.
— The Vigilant Fox 🦊 (@VigilantFox) February 16, 2022
Dr. Malone: "There appears that there may be a major financial conflict of interest on the part of Mr. Trudeau." pic.twitter.com/HWPebJXWRv
During his appearance on Tommy’s Podcast alongside Dr. Pierre Kory in February 2022, Dr. Robert Malone said the following:
“There is, in Canada, quite a bit of speculation that Justin Trudeau and his family’s foundation holds 40% of Acuitas. Acuitas is the manufacturer of the [catitic?] lipids that are used by Pfizer, and the formulation technology. It’s privately held, so there appears that there may be a major financial conflict of interest on the part of Mr. Trudeau.”
– Robert Malone
Dr. Kory interjects: “You’ve gotta be kidding me. How long have you known this, Robert?”
It’s actually a really good question. Dr. Malone, by his account and by historic record, is well-versed in the history of the development of the tech given that he played a big part in kicking it off. Not that he would be familiar with the inner workings of Acuitas, but Dr. Kory’s frustrated incredulity could even be interpreted as being directed towards Malone himself. It’s as if he is suggesting Malone has kept something big from the world.
Malone replies, “I have known that there was speculation that this might be the case… remember, I know Pieter Cullis, the academic at the University of British Columbia that gave rise to Acuitas, and I’ve spoken to him over time, including over the last couple of years a couple of times… I’ve known him professionally for decades. He would not take my calls last night when I was seeking to verify this information about ownership. So there’s financial COI issues that are going to come to fore.”
He didn’t answer the question at all, and changes the subject to safety issues with the LNPs. I don’t find his answer credible at all — no more so that the fact checks that came out dismissing his claims after the fact. At least Reuters was able to reach Cullis and crew for comment. If Justin Trudeau does hold financial interest in Acuitas, it has not be demonstrated by anyone. This is strictly rumour, and frankly, I don’t like the way Dr. Malone handled this.
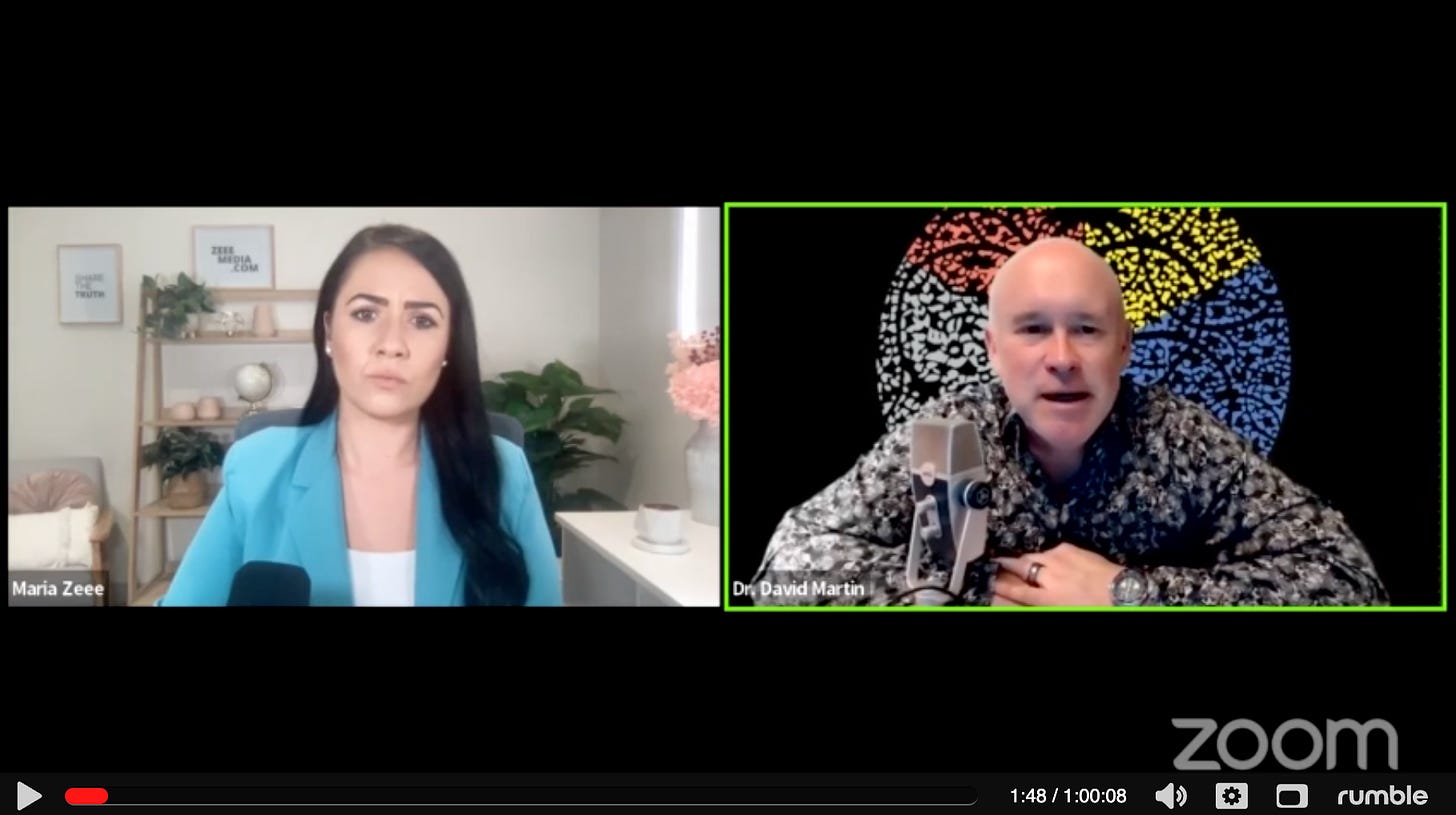
Asked by Maria Zeee about Malone’s assertion in an interview that same week, Dr. Martin acknowledged that it’s “difficult to verify” Acuitas’ books.118
What makes the official owning of who owns Acuitas difficult is they do not report in any public market. So all you can do is by inference go back and try to piece together when what happened.
Which is precisely what I have done in this article! Martin emphasizes, “the point doesn’t change. Trudeau is presiding over an illegal monopoly. He is allowing two competitors to price fix an extortion on the world. And that’s an illegal thing to do no matter who owns what.”
Conflict of Interest
The question is this: does the Government of Canada, as a result of its many long-term business development relationships specifically in the realm of research and development, hold a financial stake in the resulting intellectual property that wound up in either BNT162b2 or mRNA-1273?
Keep in mind that this is not at all an absurd premise. As described in the case of the pseudouridine patents filed by Katalin Karikó and Derrick Rossi, the United States government is a co-owner because they were developed through funding from the National Institutes of Health. This is because of the Bayh-Dole Act, and it means the U.S. government is a financial beneficiary of the COVID-19 vaccine campaign. I’m proposing this is likely the case with the Canadian government, too. In fact, Knowledge Ecology International (KEI) pointed out a provision of law on the books described in the “Policy on Title to Intellectual Property Arising Under Crown Procurement Contracts” which allows the government to “exercise rights in patents arising under procurement contracts in Canada.”119 Did this, or a similar provision or funding arrangement, result in royalties to the Government of Canada upon licensure of the LNP patents for use in COVID-19 vaccine products?

As previously described, both sides of the Acuitas/Arbutus dynasty were recipients of funding from the Government of Canada.
Inex Pharmaceuticals was granted $7+ million in 1999, which carried through all the company’s reorganizations and name changes to Tekmira and then Arbutus BioPharma. Protiva received a $150,000 grant in 2012, with AlCana/Acuitas receiving just shy of $1 million from 2011-2015.

Additionally, one of Protiva’s investors, BDC Venture Capital, is itself an investment arm of the Government’s Business Development Bank of Canada, which also holds investments in another Protiva investor, Lumira Ventures.120
The program through which Inex was incubated, Technology Partnerships Canada, boasted in its 1999 annual report that it was “keenly aware that the investments we make today will bring economic returns tomorrow.”121

Inex Pharmaceuticals is proudly featured as a government investment in TPC’s annual report from 2000, as well. The report describes “INEX’s unique delivery technology, called Transmembrane Carrier System (TCS), which enables [drugs] to live longer in the bloodstream and increases their ability to reach disease sites.”122 TCS is the term used to describe the liposome-lipid nanoparticle technology in its earliest days of development.
If it is the case that these funding programs include any form of royalty share, then it stands to reason that the Government of Canada held a conflict of interest in its entire response to the COVID-19 “pandemic.” If the government stood to benefit financially from the deployment of decades worth of patented tech development, then this option would have been far more preferable to less expensive, off-patent countermeasures such as repurposed hydroxychloroquine or ivermectin. I submit that such a conflict of interest could even influence public officials to avoid pursuing rather obvious health supporting measures such as supplementing vitamin D en masse, and making vitamin C, zinc, quercetin, melatonin widely available to all at low cost or for free.
Misrepresentation of the technology
The other thing that this long, well-documented history shows is that the Government of Canada was well aware of the nature of the lipid nanoparticle technology and its gene therapy applications in 2020 when Pfizer-BioNTech and Moderna both started clinical trials. Yet throughout 2020, 2021 and 2022, officials did everything they could to ridicule, dismiss, or otherwise attack any suggestion that the COVID-19 “vaccines” were anything other than vaccines. Public health leaders, academics, researchers and politicians alike all repeated mantras of “safe and effective” while describing mechanisms of action that were flat-out lies. With this, we can demonstrate that broad swaths of institutional personnel violated all kinds of laws while promoting these injections to the public, not the least of which are the internationally-protected laws of informed consent.

Given this, what if the Government of Canada was also aware that Canadians were highly unlikely to agree to take an unproven, historically dangerous and otherwise completely unfamiliar gene therapy product with no merit whatsoever to its use? Even if Canadians were afraid of catching the virus and developing COVID-19, it’s not hard to imagine that a decent proportion of the population would decline to participate in a mass experiment with known safety risks. How could one address this problem? Lock people in their homes, make them afraid for their lives and life’s work, isolate them from all contact, shower them in alcohol, fentanyl-laced drugs and legal opioids, and dehumanize them at every turn. Whether you want to call it mass formation or Stockholm syndrome, it did the job.
And I believe it was illegal coercion. If you ask David Martin, he calls it “price fixing” and “racketeering”, as the government got involved in business negotiations that were “anti-competitive” and “anti-consumer.”
ATIP questions
Here are the specific records requests I propose taking to the appropriate departments of the Government of Canada:
- All records related to the April 9, 2020 speech delivered by Prime Minister Trudeau;
- All records related to coronavirus vaccine development and/or mRNA vaccine development (for any purpose) dated between September 1, 2019 and April 9, 2020;
- All records related to federal funding/grants/investments in Inex Pharmaceuticals, Protiva Biotherapeutics, Tekmira Pharmaceuticals, AlCana Therapeutics, Acuitas Therapeutics and Arbutus Biopharma;
- All records related to royalties/other income generated by intellectual property related to lipid nanoparticle technology developed by the above companies;
- All records related to disclosures of interest or ownership in the above companies among employees or elected officials within the federal government;
- All records related to government claims on intellectual property arising from COVID-19-related procurement contracts.
Thank you for reading. This is an ongoing investigation, and I hope this has elucidated at least the broad strokes of what is known and unknown about Canada’s role in the development of these products. I will be pursuing these records requests and will share updates as I get them.
To end off this article, I’ve included a list of patents that I was able to document as being related to the development of the lipid nanoparticles and modified RNA in use in BNT162b2 and mRNA-1273, though some are indirect. I thought about putting it behind a paywall, but decided instead to simply ask you to support my work with a paid subscription or one-time support if you found this valuable.
Thank you very much!
Patents
Liposomes/lipid nanoparticles
- September 30, 1994: Polyethylene glycol modified ceramide lipids and liposome uses thereof (WO1996010391A1)
- September 30, 1994: Bilayer stabilizing components and their use in forming programmable fusogenic liposomes (CA2201121C)
- February 27, 1995: Method for loading lipid vesicles (CA2213861C)
- April 11, 1996: Fusogenic liposomes (CA2252055C)
- May 14, 1997: High efficiency encapsulation of charged therapeutic agents in lipid vesicles (CA2289702C)
- April 20, 1999: Cationic peg-lipids and methods of use (CA2370690A1)
- July 15, 1999: Methods for preparation of lipid-encapsulated therapeutic agents (CA2378438C)
- August 27, 1999: Compositions for stimulating cytokine secretion and inducing an immune response (CA2382611A1)
- October 20, 2000: Lipid formulations for target delivery (CA2426244A1)
- June 28, 2002: Method and apparatus for producing liposomes (CA2491164C)
- April 15, 2008: Novel lipid formulations for nucleic acid delivery (CA2721333C)
- May 23, 2008: Modified drugs for use in liposomal nanoparticles (CA2989616A1)
- October 9, 2008: Improved amino lipids and methods for the delivery of nucleic acids (CA2984026C)
- November 4, 2009: Nucleic acid-containing lipid particles and related methods (CA2816925A1)
- December 18, 2009: Lipid particles for delivery of nucleic acids (CA2784568A1)
- June 30, 2010: Non-liposomal systems for nucleic acid delivery (US9404127B2)
- October 25, 2011: Limit size lipid nanoparticles and related methods (CA2853316C)
- March 15, 2013: Lipid nanoparticles for transfection and related methods (CA2906732A1)
- February 24, 2015: Continuous flow microfluidic system (CA2977768A1)
- October 28, 2015: Lipids and lipid nanoparticle formulations for delivery of nucleic acids (CA3003055A1)
Pseudouridine
- August 23, 2005: RNA containing modified nucleosides and methods of use thereof (US010232055B2)
- August 23, 2005: RNA containing modified nucleosides and methods of use thereof (US009750824B2)
- August 23, 2005: RNA containing modified nucleosides and methods of use thereof (US8835108B2)
- August 23, 2005: RNA containing modified nucleosides and methods of use thereof (US8748089B2)
- August 23, 2005: RNA containing modified nucleosides and methods of use thereof (US8691966B2)
- August 23, 2005: RNA containing modified nucleosides and methods of use thereof (US8278036B2)
- December 7, 2009: RNA preparations comprising purified modified RNA for reprogramming cells (US010006007B2)
- December 7, 2009: RNA preparations comprising purified modified RNA for reprogramming cells (US009371511B2)
- December 7, 2009: RNA preparations comprising purified modified RNA for reprogramming cells (US009163213B2)
- December 7, 2009: RNA preparations comprising purified modified RNA for reprogramming cells (US009012219B2)
- September 28, 2010: Sustained polypeptide expression from synthetic, modified RNAs and uses thereof (US20120046346A1)
- September 28, 2010: Sustained polypeptide expression from synthetic, modified RNAs and uses thereof (US20120322865A1)
- September 28, 2010: Sustained polypeptide expression from synthetic, modified RNAs and uses thereof (US20120322864A1)
- September 28, 2010: Kit for making induced pluripotent stem cells using modified RNAs (US8716465B2)
- September 28, 2010: Compositions, kits, and methods for making induced pluripotent stem cells using synthetic modified RNAs (US8802438B2)
- September 28, 2010: Sustained polypeptide expression from synthetic, modified RNAs and uses thereof (US20140308746A1)
- September 28, 2010: Kits comprising linear DNAs for sustained polypeptide expression using synthetic, modified RNAs (US8883506B2)
- September 28, 2010: Induced pluripotent stem cells with synthetic modified RNAs (US9803177B2)
- September 28, 2010: Sustained polypeptide expression from synthetic, modified RNAs and uses thereof (US20180216078A1)
- September 28, 2010: Sustained polypeptide expression from synthetic, modified RNAs and uses thereof (US10344265B2)
- September 28, 2010: Sustained polypeptide expression from synthetic, modified RNAs and uses thereof (US20190382729A1)
- September 28, 2010: Isolated mammalian somatic cells containing modified RNA encoding OCT4, SOX2, and KLF4 (US11186829B2)
- September 28, 2010: Sustained polypeptide expression from synthetic, modified RNAs and uses thereof (US20220162565A1)
- April 4, 2011: Efficient protein expression in vivo using modified RNA (mod-RNA) (US20140073687A1)
- April 4, 2011: Efficient protein expression in vivo using modified RNA (MOD-RNA) (US10086043B2)
- April 4, 2011: Efficient protein expression in vivo using modified RNA (MOD-RNA) (US20190117733A1)
- April 4, 2011: Efficient protein expression in vivo using modified RNA (MOD-RNA) (US20200000881A1)
- September 7, 2012: Hematopoietic stem cell specific reporter mouse and uses thereof (US20150223436A1)
- September 7, 2012: Hematopoietic stem cell specific reporter mouse and uses thereof (US10080354B2)
- March 14, 2013: Compositions and methods for reprogramming hematopoietic stem cell lineages (US20160032317A1)
- April 4, 2013: Therapeutic uses of genome editing with CRISPR/Cas systems (US20150071889A1)
- April 4, 2013: Therapeutic uses of genome editing with CRISPR/Cas systems (US20150176013A1)
- April 4, 2013: Method of making a deletion in a target sequence in isolated primary cells using Cas9 and two guide RNAs (US9822370B2)
- April 4, 2013: Therapeutic uses of genome editing with CRISPR/Cas systems (US20180291383A1)
- April 4, 2013: Therapeutic uses of genome editing with CRISPR/Cas systems (US20220333119A1)
- July 9, 2013: Therapeutic uses of genome editing with CRISPR/Cas systems (US20210285015A1)
- August 23, 2013: Therapeutic uses of genome editing with CRISPR/Cas systems (US20150152436A1)
- August 23, 2013: Therapeutic uses of genome editing with CRISPR/Cas systems (US10208319B2)
- August 23, 2013: Therapeutic uses of genome editing with CRISPR/Cas systems (US20200048659A1)
- August 23, 2013: Therapeutic uses of genome editing with CRISPR/Cas systems (US20210274726A1)
- August 23, 2013: Therapeutic uses of genome editing with CRISPR/Cas systems (US20210277423A1)
- April 6, 2015: Compositions and methods for non-myeloablative conditioning (US20200148776A1)
- April 6, 2015: Compositions and methods for non-myeloablative conditioning (US10906980B2)
- April 6, 2015: Compositions and methods for non-myeloablative conditioning (US20210388094A1)
- June 25, 2015: Methods and compositions relating to hematopoietic stem cell expansion, enrichment, and maintenance (US20200248143A1)
- August 19, 2015: Methods and compositions relating to hematopoietic stem cell expansion, enrichment, and maintenance (US20180187156A1)
- August 19, 2015: Methods and compositions relating to hematopoietic stem cell expansion, enrichment, and maintenance (US10669528B2)
- August 28, 2015: Method for reducing immunogenicity of RNA (US010808242B2)
- October 9, 2015: Compositions and methods for non-myeloablative conditioning (US20190100593A1)
- October 9, 2015: Compositions and methods for non-myeloablative conditioning (US10280225B2)
- March 16, 2016: Methods and compositions relating to hematopoietic stem cell expansion (US20190119642A1)
- April 1, 2016: Methods and compositions relating to homology-directed repair (US20200149038A1)
- April 6, 2016: Compositions and methods for non-myeloablative conditioning (US10570207B2)
- March 31, 2017: Antibody-mediated conditioning with immunosuppression to enable allogenic transplantation (US20200188527A1)
Vaccines
- April 22, 2020: Coronavirus vaccine (WO2021213945A1)
References
- Liposomes and Lipid Nanoparticles as Delivery Vehicles for Personalized Medicine. (2018, November 16). Exelead Biopharma. http://archive.today/2021.07.24-200023/https://www.exeleadbiopharma.com/news/liposomes-and-lipid-nanoparticles-as-delivery-vehicles-for-personalized-medicine ↩︎
- Gao, W., Hu, C.-M. J., Fang, R. H., & Zhang, L. (2013). Liposome-like nanostructures for drug delivery. Journal of Materials Chemistry B, 1(48), 6569. https://doi.org/10.1039/c3tb21238f ↩︎
- Shade C. W. (2016). Liposomes as Advanced Delivery Systems for Nutraceuticals. Integrative medicine (Encinitas, Calif.), 15(1), 33-36. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4818067/ ↩︎
- Malone, R. W., Felgner, P. L., & Verma, I. M. (1989). Cationic liposome-mediated RNA transfection. Proceedings of the National Academy of Sciences of the United States of America, 86(16), 6077–6081. https://doi.org/10.1073/pnas.86.16.6077 ↩︎
- Malone, R. W. (1989). mRNA transfection of cultured eukaryotic cells and embryos using cationic liposomes. Focus, 11(4), 61-66. https://web.archive.org/web/20220115231218/https://static1.squarespace.com/static/550b0ac4e4b0c16cdea1b084/t/ 60a50c6e2963a366953cb517/1621429359059/K. +Focus+mRNA+Transfect+of+Cultured+Euk+Cell+embryos+1989+Data+198820210212.pdf ↩︎
- Wolff, J., Malone, R., Williams, P., Chong, W., Acsadi, G., Jani, A., & Felgner, P. (1990). Direct gene transfer into mouse muscle in vivo. Science, 247(4949), 1465–1468. https://doi.org/10.1126/science.1690918 ↩︎
- Felgner, P., Wolff, J., Rhodes, G., Malone, R., & Carson, D. (1989, March 21). Lipid-mediated polynucleotide administration to deliver a biologically active peptide and to induce a cellular immune response. Google Patents. https://patents.google.com/patent/US20030032615A1/en ↩︎
- Felgner, P. L., Wolff, J. A., Rhodes, G. H., Malone, R. W., & Carson, D. A. (1989, March 21). Lipid-mediated polynucleotide administration to reduce likelihood of subject’s becoming infected. Google Patents. https://patents.google.com/patent/US6867195B1/en ↩︎
- Felgner, P. L., Wolff, J. A., Rhodes, G. H., Malone, R. W., & Carson, D. A. (1989, March 21). Induction of a protective immune response in a mammal by injecting a DNA sequence. Google Patents. https://patents.google.com/patent/US6214804B1/en ↩︎
- The Pink Sheet. (1991, June 17). Merck/Vical. Pharma Intelligence; Informa. http://archive.today/2022.03.20-002836/https://scrip.pharmaintelligence.informa.com/PS019333/MerckVical ↩︎
- Martinon, F., Krishnan, S., Lenzen, G., Magné, R., Gomard, E., Guillet, J.-G., Lévy, J.-P., & Meulien, P. (1993). Induction of virus- specific cytotoxic T lymphocytesin vivo by liposome-entrapped mRNA. European Journal of Immunology, 23(7), 1719–1722. https://doi.org/10.1002/eji.1830230749 ↩︎
- Jirikowski, G. F., Sanna, P. P., Maciejewski-Lenoir, D., & Bloom, F. E. (1992). Reversal of Diabetes Insipidus in Brattleboro Rats: Intrahypothalamic Injection of Vasopressin mRNA. Science, 255(5047), 996–998. https://doi.org/10.1126/science.1546298 ↩︎
- Kutzler, M. A., & Weiner, D. B. (2008). DNA vaccines: ready for prime time? Nature Reviews Genetics, 9(10), 776–788. https://doi.org/10.1038/nrg2432 ↩︎
- Conry, R. M., LoBuglio, A. F., Wright, M., Sumerel, L., Pike, M. J., Johanning, F., Benjamin, R., Lu, D., & Curiel, D. T. (1995). Characterization of a messenger RNA polynucleotide vaccine vector. Cancer research, 55(7), 1397–1400. https://pubmed.ncbi.nlm.nih.gov/7882341/ ↩︎
- Boczkowski, D., Nair, S. K., Snyder, D., & Gilboa, E. (1996). Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. The Journal of experimental medicine, 184(2), 465–472. https://doi.org/10.1084/jem.184.2.465 ↩︎
- Hoerr, I., Obst, R., Rammensee, H.-G., & Jung, G. (2000). In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. European Journal of Immunology, 30(1), 1–7. https://pubmed.ncbi.nlm.nih.gov/10602021/ ↩︎
- Heiser, A., Coleman, D., Dannull, J., Yancey, D., Maurice, M. A., Lallas, C. D., Dahm, P., Niedzwiecki, D., Gilboa, E., & Vieweg, J. (2002). Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. Journal of Clinical Investigation, 109(3), 409–417. https://doi.org/10.1172/jci0214364 ↩︎
- DeFrancesco, L. (2017). The “anti-hype” vaccine. Nature Biotechnology, 35(3), 193–197. https://doi.org/10.1038/nbt.3812 ↩︎
- Karikó, K., Kuo, A., Barnathan, E. S., & Langer, D. J. (1998). Phosphate-enhanced transfection of cationic lipid-complexed mRNA and plasmid DNA. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1369(2), 320–334. https://doi.org/10.1016/s0005-2736(97)00238-1 ↩︎
- Karikó, K., Kuo, A., & Barnathan, E. S. (1999). Overexpression of urokinase receptor in mammalian cells following administration of the in vitro transcribed encoding mRNA. Gene Therapy, 6(6), 1092–1100. https://doi.org/10.1038/sj.gt.3300930 ↩︎
- Penn study finds a new role for RNA in human immune response. (2005, August 23). PENN Medicine. https://web.archive.org/web/20210421113722/https://www.pennmedicine.org/news/news-releases/2005/august/penn-study-finds-a-new-role ↩︎
- Karikó, K., Ni, H., Capodici, J., Lamphier, M., & Weissman, D. (2004). mRNA Is an Endogenous Ligand for Toll-like Receptor 3. Journal of Biological Chemistry, 279(13), 12542–12550. https://doi.org/10.1074/jbc.m310175200 ↩︎
- Karikó, K., Buckstein, M., Ni, H., & Weissman, D. (2005). Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity, 23(2), 165–175. https://doi.org/10.1016/ j.immuni.2005.06.008 ↩︎
- Office of Research & Innovation. (2020, April 28). Bayh-Dole Act. Drexel University. http://archive.today/2021.08.18-100743/https://drexel.edu/research/innovation/technology-commercialization/bayh-dole-act/ ↩︎
- Abinader, L. G. (2020, November 30). Foundational mRNA patents are subject to the Bayh-Dole Act provisions. Knowledge Ecology International. http://archive.today/2022.03.20-235410/https://www.keionline.org/34733 ↩︎
- Allen, A. (2020, November 22). Government-funded scientists laid the groundwork for billion-dollar vaccines. American Society for Biochemistry and Molecular Biology. http://archive.today/2022.03.21-192416/https://www.asbmb.org/asbmb-today/science/112220/government-funded-scientists-laid-the-groundwork-f ↩︎
- Magyar kutató, hányatatott sorsú szabadalma is kellett a most bejelentett vakcinához. (2020, November 9). Forbes. http://archive.today/2022.03.21-192812/https://forbes.hu/uzlet/hanyatatott-sorsu-magyar-szabadalom-is-kellett-a-most-bejelentett-vakcinahoz/ ↩︎
- Warren, L., Manos, P. D., Ahfeldt, T., Loh, Y.-H., Li, H., Lau, F., Ebina, W., Mandal, P. K., Smith, Z. D., Meissner, A., Daley, G. Q., Brack, A. S., Collins, J. J., Cowan, C., Schlaeger, T. M., & Rossi, D. J. (2010). Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell, 7(5), 618–630. https://doi.org/10.1016/j.stem.2010.08.012 ↩︎
- Walrath, R. (2022, March 16). How Moderna helped RNA drugs come of age. BizJournals; Boston Business Journal. http://archive.today/2022.03.16-200517/https://www.bizjournals.com/boston/news/2022/03/16/how-moderna-helped-rna-drugs-come-of-age.html ↩︎
- Piper, E. (2021, July 20). Scientists vs Science: Interviews with Mike Yeadon and Robert Malone. Logically. http://archive.today/2021.07.22-012742/https://www.logically.ai/articles/scientists-vs-science-interviews-with-mike-yeadon-and-robert-malone ↩︎
- Horejs, C. (2021). From lipids to lipid nanoparticles to mRNA vaccines. Nature Reviews Materials. https://doi.org/10.1038/s41578-021-00379-9 ↩︎
- Technology Overview. (2000, June 4). Inex Pharmaceuticals. http://archive.today/2000.06.04-233753/http://inexpharm.com/technology_frame.html ↩︎
- Kulkarni, J. A., Cullis, P. R., & van der Meel, R. (2018). Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Therapeutics, 28(3), 146–157. https://doi.org/10.1089/nat.2018.0721 ↩︎
- Choi, L. S. L., Madden, T. D., & Webb, M. S. (1994, September 30). Polyethylene glycol modified ceramide lipids and liposome uses thereof. Canada Patent Database. http://archive.today/2022.03.08-204712/https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2201120/summary.html?type=number_search&tabs1Index=tabs1_1 ↩︎
- Madden, T. D., Cullis, P. R., & Holland, J. W. (1994). Bilayer stabilizing components and their use in forming programmable fusogenic liposomes (Canadian Intellectual Property Office Patent No. 2201121). http://archive.today/2022.03.08-204044/https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2201121/summary.html?type=number_search&tabs1Index=tabs1_1 ↩︎
- Cullis, P. R., Fenske, D. B., Hope, M. J., & Wong, K. F. (1995, February 27). Method for loading lipid vesicles (Canadian Intellectual Property Office Patent No. CA 2213861). Canadian Patent Database. http://archive.today/2022.03.08-213848/https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2213861/summary.html?type=number_search&tabs1Index=tabs1_1 ↩︎
- Cullis, P. R., Choi, L. S. L., Monck, M., & Bailey, A. (1996, April 11). Fusogenic liposomes (Canadian Intellectual Property Office Patent No. CA 2252055). Canadian Patent Database. http://archive.today/2022.03.08-215546/https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2252055/summary.html?type=number_search&tabs1Index=tabs1_1 ↩︎
- Cullis, P. R., Fenske, D. B., & MacLachlan, I. (2000, October 25). Lipid formulations for target delivery (Canadian Intellectual Property Office Patent No. CA 2426244). Canadian Patent Database. http://archive.today/2022.03.08-235645/https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2426244/summary.html?type=number_search&tabs1Index=tabs1_1 ↩︎
- Vardi, N. (2021, August 17). COVID’s forgotten hero: the untold story of the scientist whose breakthrough made the vaccines possible. Forbes. http://archive.today/2022.03.22-150124/https://www.forbes.com/sites/nathanvardi/2021/08/17/covids-forgotten-hero-the-untold-story-of-the-scientist-whose-breakthrough-made-the-vaccines-possible/?sh=5855d68a354f ↩︎
- FOI Request – HTH-2015-51828. (2016, February 12). Government of British Columbia. https://web.archive.org/web/20220428045844/http://docs.openinfo.gov.bc.ca/Response_Package_HTH-2015-51828.pdf ↩︎
- Zimmermann, T. S., Lee, A. C. H., Akinc, A., Bramlage, B., Bumcrot, D., Fedoruk, M. N., Harborth, J., Heyes, J. A., Jeffs, L. B., John, M., Judge, A. D., Lam, K., McClintock, K., Nechev, L. V., Palmer, L. R., Racie, T., Röhl, I., Seiffert, S., Shanmugam, S., & Sood, V. (2006). RNAi-mediated gene silencing in non-human primates. Nature, 441(7089), 111–114. https://doi.org/10.1038/ nature04688 ↩︎
- Garcia, J. A. (2001, December 13). Inex Pharmaceuticals completes bought deal of common shares ($43 million). Lexpert. http://archive.today/2022.03.22-140807/https://www.lexpert.ca/big-deals/inex-pharmaceuticals-completes-bought-deal-of-common-shares-43-million/345416 ↩︎
- 2004 Annual Report. (2004). Inex Pharmaceuticals Corporation. https://web.archive.org/web/20220322135752/https://doc.morningstar.com/Document/2ded6a03c7093ab6.msdoc/original?clientid=globaldocuments&key=52dbc583e1012395 ↩︎
- Inex Pharmaceuticals slashes more staff as CEO departs. (2005, June 21). CBC News; CBC/Radio-Canada. http://archive.today/2022.03.22-140227/https://www.cbc.ca/news/business/inex-pharmaceuticals-slashes-more-staff-as-ceo-departs-1.525609 ↩︎
- INEX Pharmaceuticals provides timeline for completing spin-out of Tekmira Pharmaceuticals Corporation. (2007, February 26). BioSpace. http://archive.today/2022.03.22-140608/https://www.biospace.com/article/releases/inex-pharmaceuticals-provides-timeline-for-completing-spin-out-of-tekmira-pharmaceuticals-corporation-/ ↩︎
- Inex Pharmaceuticals announces completion of spin-out of Tekmira Pharmaceuticals Corporation. (2007, May 1). Arbutus Bio. https://web.archive.org/web/20230830184647/https://investor.arbutusbio.com/static-files/7043d792-acbb-47ae-9b0f-ce74445f583a ↩︎
- Protiva Biotherapeutics – Funding, Financials, Valuation & Investors. Crunchbase. Retrieved February 12, 2023, from https://www.crunchbase.com/organization/protiva-biotherapeutics/company_financials ↩︎
- Tekmira Pharmaceuticals Corporation. Business Development Bank of Canada. Retrieved February 12, 2023, from http://archive.today/2023.02.12-232323/https://www.bdc.ca/en/bdc-capital/venture-capital/portfolio/tekmira-pharmaceuticals-corporation ↩︎
- Tekmira Pharmaceuticals Corporation and Protiva Biotherapeutics announce business combination to create new company focused on RNAi delivery and therapeutics. (2008, March 31). Fierce Biotech. http://archive.today/2022.03.22-142058/https://www.fiercebiotech.com/biotech/tekmira-pharmaceuticals-corporation-and-protiva-biotherapeutics-announce-business ↩︎
- Lindenboom, C. R., & Brodsky, J. (2021, January 10). Alnylam launches “Alnylam P5x25” strategy for planned transition to a top five biotech in market capitalization over next five years. Business Wire. http://archive.today/2022.03.22-160320/https://www.businesswire.com/news/home/20210110005034/en/Alnylam-Launches-%E2%80%9CAlnylam-P5x25%E2%80%9D-Strategy-for-Planned-Transition-to-a-Top-Five-Biotech-in-Market-Capitalization-Over-Next-Five-Years ↩︎
- Investigations of nanoparticulate formulations of lipophilic prodrugs. (2008, November 20). Canadian Institutes of Health Research. https://web.archive.org/web/20230213024913/https://webapps.cihr-irsc.gc.ca/decisions/p/project_details.html?applId=183810&lang=en ↩︎
- About Us. Acuitas Therapeutics. Retrieved March 22, 2022, from http://archive.today/2022.02.14-032459/https://acuitastx.com/company/our-journey/ ↩︎
- Exhibit 10.2. (2009, July 27). Real Deal Docs. https://agreements.realdealdocs.com/Addendum-or-Modifications/Alnylam-Tekmira-Protiva-UBC-AlCa-2894169/ ↩︎
- GlobeNewswire. (2011, June 3). Tekmira files amended complaint in Alnylam litigation. NBC News. http://archive.today/2022.03.22-142219/https://www.nbcnews.com/id/wbna43273972 ↩︎
- Treasury Board of Canada Secretariat. (2011, July 11). Grants and Contributions – GC-2011-Q2-18966. Open Government Portal. http://archive.today/2022.05.19-003225/https://search.open.canada.ca/grants/record/nrc-cnrc,GC-2011-Q2-18966,current ↩︎
- Producing lipid nanoparticle technology for vaccine delivery (Acuitas Therapeutics). (2021, May 17). National Research Council Canada. https://web.archive.org/web/20210518160032/https://nrc.canada.ca/en/stories/producing-lipid-nanoparticle-technology-vaccine-delivery-acuitas-therapeutics ↩︎
- Treasury Board of Canada Secretariat. (2012, July 16). Grants and Contributions – GC-2012-Q4-21817. Open Government Portal. http://archive.today/2022.05.19-003524/https://search.open.canada.ca/grants/record/nrc-cnrc,GC-2012-Q4-21817,current ↩︎
- Treasury Board of Canada Secretariat. (2012, July 16). Grants and Contributions – GC-2012-Q2-20144. Open Government Portal. http://archive.today/2022.05.19-003749/https://search.open.canada.ca/grants/record/nrc-cnrc,GC-2012-Q2-20144,current ↩︎
- Grants and Contributions – GC-2013-Q3-23969. (2013, September 13). Government of Canada. http://archive.today/2022.03.08-211702/https://search.open.canada.ca/en/gc/id/nrc-cnrc,GC-2013-Q3-23969,current ↩︎
- Grants and Contributions – GC-2014-Q4-26252. (2015, February 6). Government of Canada. http://archive.today/2022.03.08-212018/https://search.open.canada.ca/en/gc/id/nrc-cnrc,GC-2014-Q4-26252,current ↩︎
- Funding Decisions Database. Canadian Institutes of Health Research. Retrieved February 13, 2023, from https://webapps.cihr-irsc.gc.ca/decisions/p/main.html?lang=en#fq= ↩︎
- Treasury Board of Canada Secretariat. (2012, April 20). GC-2012-Q1-19934. Open Government Portal. http://archive.today/2022.05.19-002256/https://search.open.canada.ca/grants/record/nrc-cnrc,GC-2012-Q1-19934,current ↩︎
- Arbutus BioPharma Corporation. (2015, September 30). Form 10-Q. Securities and Exchange Commission. https://web.archive.org/web/20171208092809/https://www.sec.gov/Archives/edgar/data/1447028/000106299315005809/form10q.htm ↩︎
- Oligonucleotide – an overview. ScienceDirect Topics. Retrieved February 12, 2023, from https://web.archive.org/web/20230212225931/https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/oligonucleotide ↩︎
- Roberts, T. C., Langer, R., & Wood, M. J. A. (2020). Advances in oligonucleotide drug delivery. Nature Reviews. Drug Discovery, 19, 1–22. https://doi.org/10.1038/s41573-020-0075-7 ↩︎
- Tekmira and Alnylam restructure relationship and settle all litigation. (2012, November 12). U.S. Securities and Exchange Commission. https://web.archive.org/web/20220322141914/https://www.sec.gov/Archives/edgar/data/1447028/000117184312004102/newsrelease.htm ↩︎
- George, J. (2017, February 10). Doylestown biopharm firm granted injunction over licensing dispute. American City Business Journals. https://web.archive.org/web/20170214043742/http://www.bizjournals.com/philadelphia/news/2017/02/10/ arbutus-biopharma-abus-injunction-acuitus-sales.html ↩︎
- Biotech stock in spotlight: as the world awaits Ebola treatment, Tekmira Pharma in prime focus. TechNews. Retrieved March 22, 2022, from https://web.archive.org/web/20210527095559/http://technews.org/biotech-stock-in-spotlight-as-the-world-awaits-ebola-treatment-tekmira-pharma-remains-in-orime-focus/2913113/ ↩︎
- Pollack, A. (2015, June 19). Clinical trial of experimental Ebola drug is halted. The New York Times. http://archive.today/2019.12.31-145430/https://www.nytimes.com/2015/06/20/health/clinical-trial-of-experimental-ebola-drug-is-halted.html ↩︎
- Cutler, A., & Baradarani, H. (2015, July 20). Tekmira announces launch of Arbutus Biopharma, a Hepatitis B solutions company. U.S. Securities and Exchange Commission. http://archive.today/2022.03.22-200915/https://www.sec.gov/Archives/edgar/data/1447028/000117184315003907/newsrelease.htm ↩︎
- Lee, A. C. H., & Weber, N. D. (2020, April 21). Delivering CRISPR therapeutics with lipid nanoparticles. Google Patents. https://patents.google.com/patent/US10626393B2/en ↩︎
- Garde, D. (2015, July 20). Tekmira changes its name and shuffles away from Ebola. Fierce Biotech. http://archive.today/2022.03.22-143605/https://www.fiercebiotech.com/r-d/tekmira-changes-its-name-and-shuffles-away-from-ebola ↩︎
- Tolmie, T., & Schull, D. (2018, April 11). Arbutus and Roivant Launch Genevant Sciences with Industry-Leading Platform to Develop Broad Range of RNA Therapeutics for Genetic Diseases. Arbutus Biopharma. https://web.archive.org/web/20230207071630/https://investor.arbutusbio.com/news-releases/news-release-details/arbutus-and-roivant-launch-genevant-sciences-industry-leading ↩︎
- Reuters Staff. (2016, October 5). Alnylam ends development of drug due to patient deaths in trial. Reuters. https://web.archive.org/web/20181129214036/https://www.reuters.com/article/us-alnylam-study-idUSKCN1252M1 ↩︎
- Rossi, D., & Warren, L. (2012, February 23). Sustained polypeptide expression from synthetic, modified RNAs and uses thereof. United States Patent and Trademark Office. https://web.archive.org/web/20230207044248/https://image-ppubs.uspto.gov/dirsearch-public/print/downloadPdf/20120046346 ↩︎
- Warren, L., Manos, P. D., Ahfeldt, T., Loh, Y.-H., Li, H., Lau, F., Ebina, W., Mandal, P. K., Smith, Z. D., Meissner, A., Daley, G. Q., Brack, A. S., Collins, J. J., Cowan, C., Schlaeger, T. M., & Rossi, D. J. (2010). Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell, 7(5), 618–630. https://doi.org/10.1016/j.stem.2010.08.012 ↩︎
- Bratzler, R. (2009, February 25). Selecta Biosciences, Inc. Closes $15.1M Series B Financing. Flagship Pioneering. http://archive.today/2023.02.03-174945/https://www.flagshippioneering.com/press/selecta-biosciences-inc-closes-151m-series-b-financing ↩︎
- Dolgin, E. (2015). Business: The billion-dollar biotech. Nature, 522(7554), 26–28. https://doi.org/10.1038/522026a ↩︎
- Garde, D. (2020, November 10). The story of mRNA: How a once-dismissed idea became a leading technology in the COVID vaccine race. STAT. http://archive.today/2021.09.14-034008/https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/ ↩︎
- Huang, G. T. (2012, December 6). Xconomy: Moderna, $40M in Tow, Hopes to Reinvent Biotech with “Make Your Own Drug.” Xconomy. http://archive.today/2020.11.25-195641/https://xconomy.com/boston/2012/12/06/moderna-40m-in-tow-hopes-to-reinvent-biotech-with-new-protein-drugs/ ↩︎
- Kutz, E. (2010, October 4). Xconomy: ModeRNA, Stealth Startup Backed By Flagship, Unveils New Way to Make Stem Cells. Xconomy. http://archive.today/2021.03.16-115008/https://xconomy.com/boston/2010/10/04/moderna-stealth-startup-backed-by-flagship-unveils-new-way-to-make-stem-cells/ ↩︎
- Moderna, Inc. (2018, November 9). S-1. Securities and Exchange Commission. http://archive.today/2022.02.20-165037/https://www.sec.gov/Archives/edgar/data/1682852/000119312518323562/d577473ds1.htm ↩︎
- Cross, R. (2019, February 20). Moderna and AstraZeneca’s mRNA therapy for heart regeneration passes Phase I safety test. Chemical & Engineering News. http://archive.today/2022.03.16-202739/https://cen.acs.org/business/Moderna-AstraZenecas-mRNA-therapy-heart/97/i8 ↩︎
- McConaghie, A. (2017, July 27). Alexion cuts ties with Moderna and re-focuses. Pharma Phorum. https://web.archive.org/web/20211025065805/https://pharmaphorum.com/news/alexion-cuts-ties-moderna-re-focuses/ ↩︎
- Garde, D. (2017, January 10). Moderna hits safety problems in bold bid to reinvent medicine. STAT. http://archive.today/2022.01.16-212432/https://www.statnews.com/2017/01/10/moderna-trouble-mrna/ ↩︎
- Servick, K. (2020, March 25). This mysterious $2 billion biotech is revealing the secrets behind its new drugs and vaccines. Science. http://archive.today/2022.01.02-004314/https://www.science.org/content/article/mysterious-2-billion-biotech-revealing-secrets-behind-its-new-drugs-and-vaccines ↩︎
- Herper, M. (2015, January 5). Moderna Therapeutics Raises $450 Million In Biggest Biotech Private Round Ever. Forbes. http://archive.today/2015.01.21-020858/http://www.forbes.com/sites/matthewherper/2015/01/05/moderna-therapeutics-raises-450-million-in-biggest-biotech-private-round-ever/ ↩︎
- CureVac Collaboration. (2015, March 5). Bill & Melinda Gates Foundation. http://archive.today/2021.04.22-200808/https://www.gatesfoundation.org/ideas/media-center/press-releases/2015/03/curevac-collaboration ↩︎
- Bill & Melinda Gates Foundation — Advancing an mRNA-based antibody combination to help prevent HIV infection. Moderna. Retrieved March 21, 2022, from http://archive.today/2021.08.23-023928/https://www.modernatx.com/ecosystem/strategic-collaborators/foundations-advancing-mrna-science-and-research ↩︎
- Weisman, R. (2016, June 29). Moderna gets $200m in expanded Merck deal. The Boston Globe. http://archive.today/2021.08.05-234717/https://www.bostonglobe.com/business/2016/06/29/moderna-gets-expanded-merck-deal/LhabVoqc45seApcHVs8xnN/story.html ↩︎
- Moderna, Inc. (2018). Form S1 (p. 186). U.S. Securities and Exchange Commission. http://archive.today/2022.02.20-165037/https://www.sec.gov/Archives/edgar/data/1682852/000119312518323562/d577473ds1.htm ↩︎
- Vardi, N. (2017, May 16). Moderna can’t escape my intellectual property, says Arbutus CEO. Forbes. http://archive.today/2022.03.22-180143/https://www.forbes.com/sites/nathanvardi/2017/05/16/moderna-cant-escape-my-intellectual-property-says-arbutus-ceo/?sh=5b6aeb01633a ↩︎
- Cutler, A., Tolmie, T., & Schull, D. (2017, April 13). Arbutus injunction survives attempted appeal by Acuitas. U.S. Securities and Exchange Commission. http://archive.today/2022.03.22-180943/https://www.sec.gov/Archives/edgar/data/1447028/000117184317002101/exh_991.htm ↩︎
- Tolmie, T., & Schull, D. (2018, February 22). Arbutus settles litigation, terminating Acuitas’ rights to LNP technology. Arbutus Biopharma. http://archive.today/2022.03.22-182650/https://investor.arbutusbio.com/news-releases/news-release-details/arbutus-settles-litigation-terminating-acuitas-rights-lnp-0 ↩︎
- Stendahl, M. (2018, February 22). Settlement clears Moderna to use Canadian firm’s drug delivery tech. American City Business Journals. http://archive.today/2021.08.25-231443/https://www.bizjournals.com/boston/news/2018/02/22/settlement-clears-moderna-to-use-canadian-firm-s.html ↩︎
- Silbersher, Z. (2018, December 17). Does Moderna Therapeutics’ pipeline depend upon its patent dispute with Arbutus Biopharma over mRNA delivery? Markman Advisors. http://archive.today/2022.03.22-180516/https://www.markmanadvisors.com/blog/2018/12/17/does-moderna-therapeutics-pipeline-depend-upon-its-patent-dispute-with-arbutus-biopharma-over-mrna-delivery ↩︎
- Exhibit 10.8. (2017, June 26). U.S. Securities and Exchange Commission. http://archive.today/2022.03.21-193128/https://www.sec.gov/Archives/edgar/data/1682852/000119312518323562/d577473dex108.htm ↩︎
- Exhibit 10.15. (2017, July 14). U.S. Securities and Exchange Commission. http://archive.today/2022.03.21-195500/https://www.sec.gov/Archives/edgar/data/1776985/000119312519241112/d635330dex1015.htm ↩︎
- Zaks, T. (2017, November). The disease-eradicating potential of gene editing. TED. https://web.archive.org/web/20211223104156/https://www.ted.com/talks/tal_zaks_the_disease_eradicating_potential_of_gene_editing ↩︎
- Arbutus Biopharma Corp management’s discussion and analysis of financial condition and results of operations (form 10-Q). (2021, November 4). MarketScreener. http://archive.today/2022.03.22-195156/https://www.marketscreener.com/quote/stock/ARBUTUS-BIOPHARMA-CORPORA-23319313/news/ARBUTUS-BIOPHARMA-CORP-MANAGEMENT-S-DISCUSSION-AND-ANALYSIS-OF-FINANCIAL-CONDITION-AND-RESULTS-OF-O-36907264/ ↩︎
- Silbersher, Z. (2020, July 28). Is Moderna’s COVID-19 vaccine using Arbutus Biopharma’s patents? Markman Advisors. http://archive.today/2022.01.12-223800/https://www.markmanadvisors.com/blog/2020/7/28/is-modernas-covid-19-vaccine-using-arbutus-biopharmas-patents ↩︎
- Kansteiner, F. (2022, February 28). After raking in billions with its COVID shot, Moderna faces patent infringement suit related to vaccine delivery tech. Fierce Pharma. http://archive.today/2022.03.22-185116/https://www.fiercepharma.com/pharma/rivals-eye-unjustified-royalties-pfizer-partner-sues-defend-covid-19-shot-comirnaty ↩︎
- Amendment No. 3 to Form F-1 Registration Statement. (2019, October 9). BioNTech; Securities and Exchange Commission. https://web.archive.org/web/20221228202950/https://investors.biontech.de/node/6751/html#toc ↩︎
- BioNTech AG. (2016, September 21). BioNTech to enter into worldwide strategic collaboration with Genentech to develop individualized mRNA cancer therapies. Cision PR Newswire. http://archive.today/2022.03.21-202352/https://www.prnewswire.com/news-releases/biontech-to-enter-into-worldwide-strategic-collaboration-with-genentech-to-develop-individualized-mrna-cancer-therapies-594231481.html ↩︎
- Cox, C., Zierke, L., Jehle, R., & Clark, M. (2015, May 12). Lilly and BioNTech announce research collaboration on novel cancer immunotherapies. BioNTech. https://web.archive.org/web/20220322010950/https://investors.biontech.de/static-files/ 0dec90b3-66df-4cee-80e7-7766b8a2a206 ↩︎
- BioNTech and Genmab sign co-development and commercialization agreement in immuno-oncology. (2015, May 19). BioNTech. http://archive.today/2022.03.22-011249/https://investors.biontech.de/news-releases/news-release-details/biontech-and-genmab-sign-co-development-and-commercialization ↩︎
- Taylor, N. P. (2016, September 21). Genentech lays $310M wager on BioNTech’s mRNA cancer vaccine platform. Fierce Biotech. http://archive.today/2022.03.21-202513/https://www.fiercebiotech.com/biotech/genentech-lays-310-million-wager-biontech-s-mrna-cancer-vaccine-platform ↩︎
- Kranz, L. M., Diken, M., Haas, H., Kreiter, S., Loquai, C., Reuter, K. C., Meng, M., Fritz, D., Vascotto, F., Hefesha, H., Grunwitz, C., Vormehr, M., Hüsemann, Y., Selmi, A., Kuhn, A. N., Buck, J., Derhovanessian, E., Rae, R., Attig, S., & Diekmann, J. (2016). Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature, 534(7607), 396–401. https://doi.org/10.1038/nature18300 ↩︎
- Dolgin, E. (2019). Unlocking the potential of vaccines built on messenger RNA. Nature, 574(7778), S10–S12. https://doi.org/10.1038/d41586-019-03072-8 ↩︎
- BioNTech and Genevant Sciences Sign Strategic mRNA-Focused Partnership in Rare Diseases. (2018, July 10). BioNTech. https://web.archive.org/web/20230314080416/https://investors.biontech.de/static-files/2e79f2f4-9513-48f3-a9ef-6acbc9868f31 ↩︎
- Alatovic, J. (2018, August 16). BioNTech Signs Collaboration Agreement with Pfizer to Develop mRNA-based Vaccines for Prevention of Influenza. BioNTech. http://archive.today/2021.12.08-155206/https://biontechse.gcs-web.com/news-releases/news-release-details/biontech-signs-collaboration-agreement-pfizer-develop-mrna-based ↩︎
- Taylor, N. P. (2019, January 4). Sanofi invests €80M in BioNTech as cancer mRNA hits clinic. Fierce Biotech. http://archive.today/2022.01.05-012610/https://www.fiercebiotech.com/biotech/sanofi-invests-eu80m-biontech-as-cancer-mrna-hits-clinic ↩︎
- Boehler, M. (2019, September 4). BioNTech announces new collaboration to develop HIV and tuberculosis programs. BioNTech. http://archive.today/2022.01.03-191438/https://investors.biontech.de/news-releases/news-release-details/biontech-announces-new-collaboration-develop-hiv-and ↩︎
- WHO Director-General’s opening remarks at the media briefing on COVID-19 – 11 March 2020. (2020, March 11). World Health Organization. ↩︎
- Erskine, M. (2020, March 18). Canada goes on COVID-19 lockdown. The Manitoulin Expositor. https://web.archive.org/web/20221126060604/https://www.manitoulin.com/canada-goes-on-COVID-19-lockdown/ ↩︎
- CBC News. (2020, April 9). “This is the new normal,” until COVID-19 vaccine developed: Trudeau. YouTube. https://web.archive.org/web/20221108025542/https://youtu.be/Qvb4WIC7Ccw ↩︎
- Vaccine Choice Canada. (2021, August 21). Dr. David E. Martin drops Shocking Covid Info on Canadians! BitChute. https://web.archive.org/web/20221130214104/https://www.bitchute.com/video/ZUVtNa9xdBnW/ ↩︎
- Dr. David Martin – Canada, Trudeau’s Illegal Monopoly, Acuitas, CRISPR, NWO & Australia – Zeee Media. (2022, February 18). Zeee Media. https://zeeemedia.com/interview/dr-david-martin-canada-trudeaus-illegal-monopoly-acuitas-crispr-nwo-australia/ ↩︎
- Abinader, L. G. (2020, April 1). Canadian Policy option that can permit the government to exercise rights in patents arising under procurement contracts in Canada, if “prior obligations” exist, and how that relates to WHO COVID-19 pooling proposal. Knowledge Ecology International. https://web.archive.org/web/20200429014737/https://www.keionline.org/32676 ↩︎
- Lumira Capital. Business Development Bank of Canada. Retrieved April 27, 2022, from http://archive.today/2022.04.28-044715/https://www.bdc.ca/en/bdc-capital/venture-capital/portfolio/lumira-capital-ii ↩︎
- Annual Report 1998-1999. Technology Partnerships Canada. Retrieved February 12, 2023, from https://web.archive.org/web/20230213000509/https://ised-isde.canada.ca/site/industrial-technologies-office/sites/default/files/attachments/tpc98-99.pdf ↩︎
- Annual Report 1999-2000. Technology Partnerships Canada. Retrieved February 12, 2023, from https://web.archive.org/web/20240314070651/https://ised-isde.canada.ca/site/industrial-technologies-office/sites/default/files/attachments/tpc_1999_2000_e.pdf ↩︎